Application of cardamom in preparation of medicine for treating and delaying intervertebral disc degenerative change
A degenerative disease and intervertebral disc technology, which is applied in the field of application of cardamomin in the preparation of drugs for treating and delaying intervertebral disc degeneration, can solve the problems of no intervertebral disc degeneration, achieve obvious anti-inflammatory and anti-oxidation effects, The effect of low dose and inhibition of inflammatory response of nucleus pulposus cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
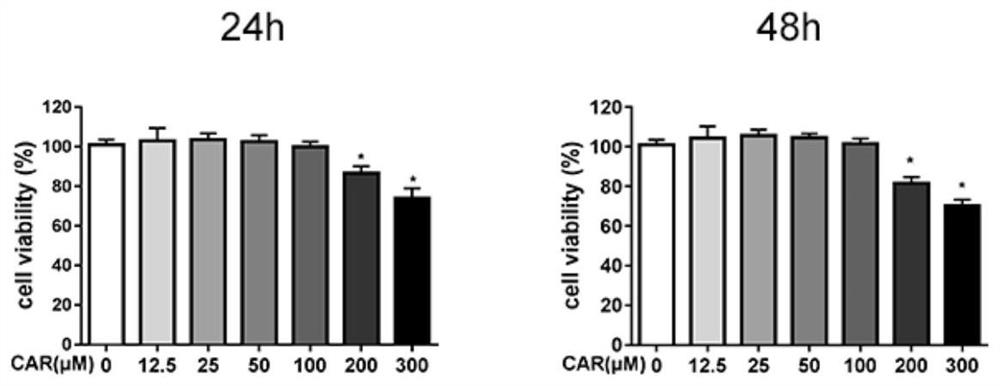
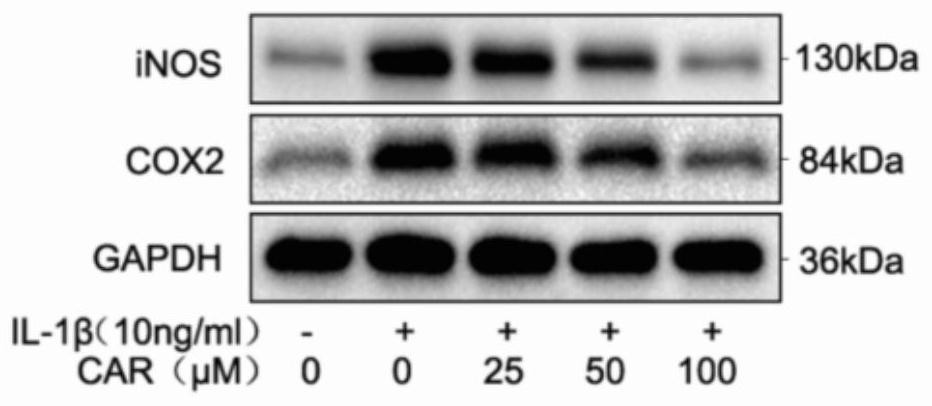
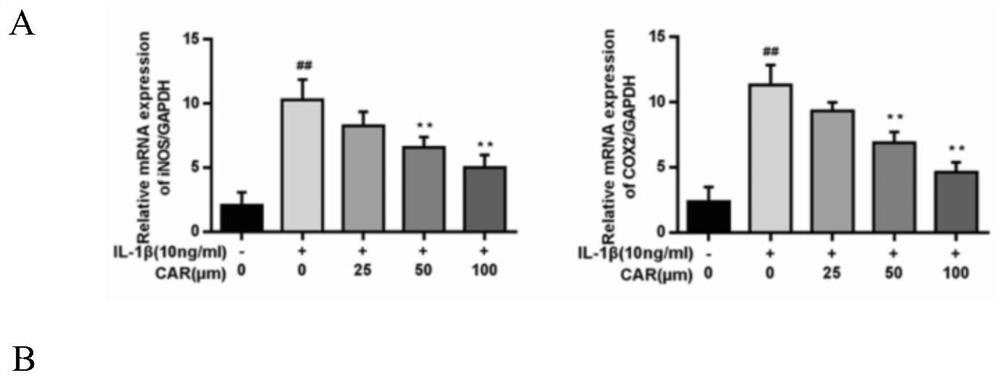
Examples
Embodiment 1
[0037] 1. Materials and reagents
[0038] Cell culture reagents (products of Life technologies): DMEM / F12 cell basal medium (10567-014, Gibco, Invitrogen, Grand Island, NY), fetal bovine serum, trypsin, 100U / ml penicillin / streptomycin (double antibody) . Other cell culture reagents: collagenase type II (Gibco).
[0039] Histological reagents (Beijing Suolaibao): hematoxylin, eosin; paraformaldehyde, paraffin, absolute ethanol, xylene, neutral resin. Cell Proliferation Detection Kit Cell Couming KIT-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan).
[0040] Western blotting and immunofluorescence reagents: Aggrecan, Collagen II, ADAMTS5, GAPDH and Lamin B1 (Abcam, Cambrige, UK), inos, COX-2, IκBα, p65, Nrf2, HO-1 and MMP13 were purchased from ProteinTech (Wuhan, China) , DAPI Fluoromount-GTM anti-fluorescence quenching mounting medium (Zhongshan Jinqiao), Goat anti-rabbit (Alexa Fluor488, Invitrogen), protein quantification kit BCAProtein Assay Kit), immunohistochemical endo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com