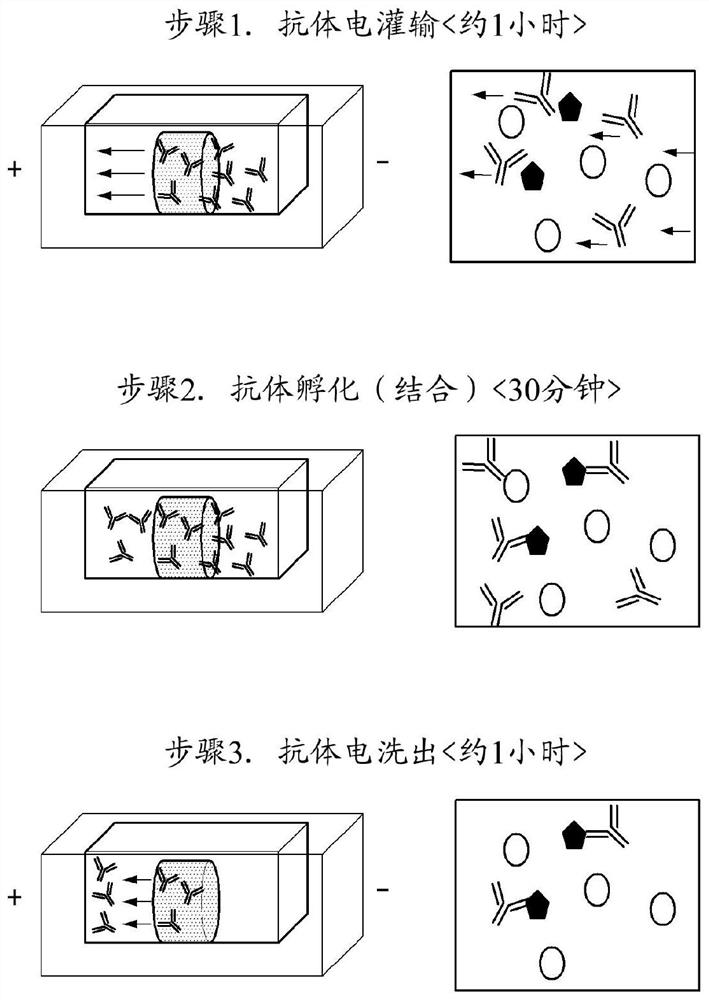
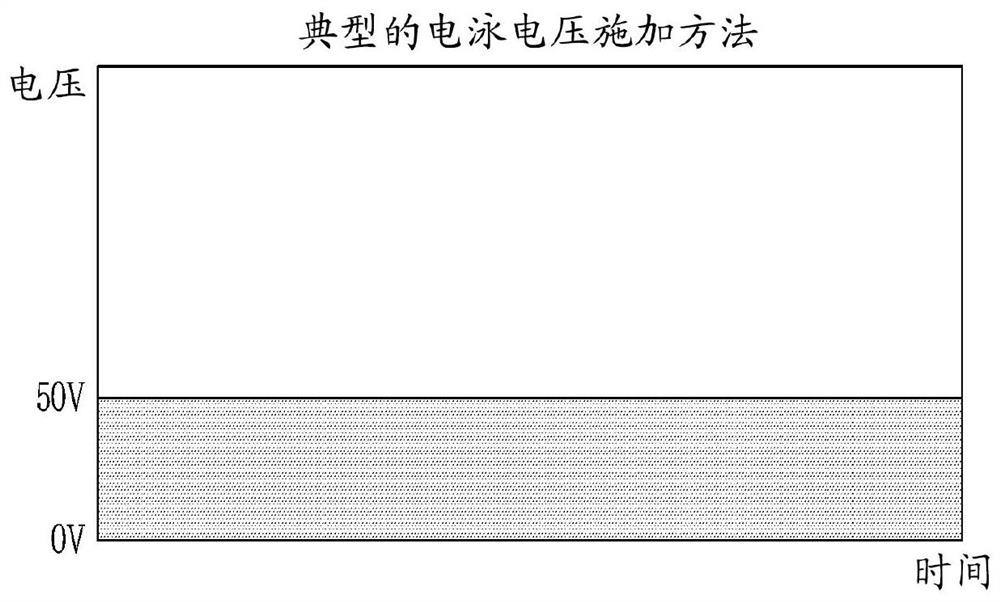
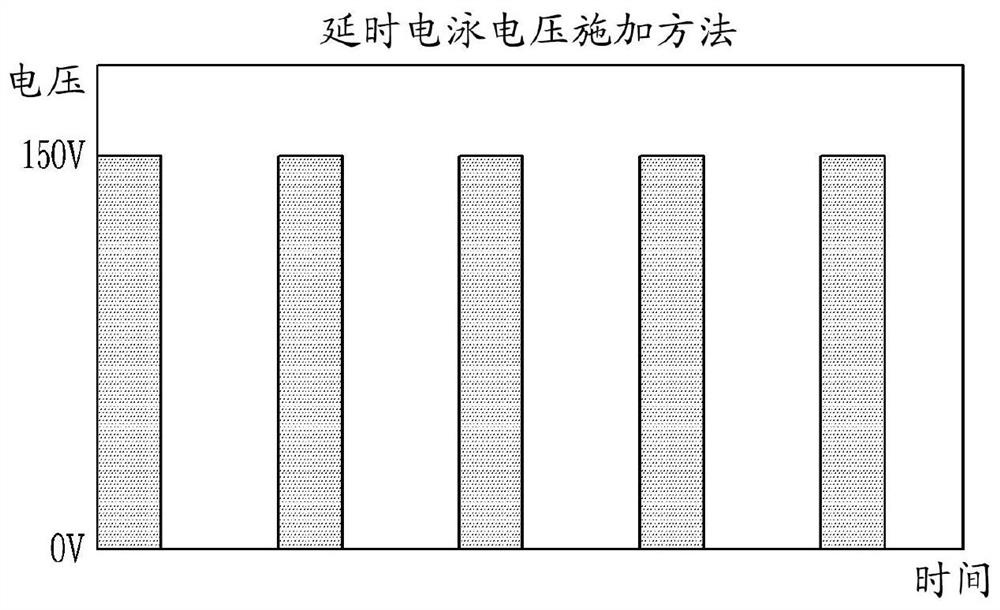
Method and apparatus for electrophoretically dyeing biological sample by using ion conductive film
An ion-conducting membrane and biological sample technology, which is applied in the preparation, sampling, and measurement devices of test samples, can solve the problems of deterioration of light scattering resolution, large numbers, and difficult internal tissue imaging, and achieve reproducible and effective staining. , High electrical efficiency, the effect of minimizing damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0146] Example 1: Preparation of samples
[0147] The brains of non-transformed rats (SD rats, 4-6 weeks old) were used to prepare clear brain tissue samples by the typical CLARITY method.
[0148] Perfusion through the heart drains blood from the microvasculature of the brain. The brain was excised from the rat, immersed in the hydrogel monomer solution, and incubated at 4°C for 2 days, wherein, in the hydrogel monomer solution, 4% (w / v) acrylamide, 0.25% (w / v) VA-044, and 4% (w / v) PFA (paraformaldehyde) were dissolved in phosphate saline (PBS) solution.
[0149] Then, the brain was placed under vacuum for 2 to 4 hours in the dark, and the temperature was raised to 37°C by using a special machine (CLARITY Easy-Imbedding, LCI).
[0150]Thereafter, after cutting the brain into the desired sample size (thickness: 500 μm, 1 mm, 1.5 mm, 2 mm, 5 mm; diameter: 5 mm, 10 mm), electrical tissue was performed by using a CLARITY machine (CLARITY Easy-Clearing, LCI) Transparency (Ele...
example 2
[0152] Example 2: Biological samples using the electrophoresis method for staining biological samples with an applied ion-conducting membrane Preparation of dyeing equipment
[0153] The fabricated sample chamber is mounted between the respective electrode parts of a device comprising a power source, two electrode parts, a buffer supply part and a cooler, each electrode part including a lower buffer inlet on one side and a buffer inlet on the opposite side. The upper part of the buffer outlet, the buffer supply part (borate buffer; 50mM LiOH, 25mM boric acid) is connected to the buffer inlet and buffer outlet of the two electrode parts, and the cooler is connected to the buffer supply part . The device includes a cooling water circulation channel arranged to completely contact the two sides, the lower surface, and the upper surface of the sample chamber except the two sides in contact with the electrodes, and has a cooling water circulation channel through which the coolin...
example 3
[0154] Example 3: Immunostaining test using ion-conducting membrane by electrophoretic technique (primary and secondary antibody detection test) - glial cell staining
[0155] CLARITY samples of brain fibers of untransformed rats (SD rats, 4-6 weeks old) obtained in Example 1 were immunized by electrophoresis using ion-conducting membranes using antibodies to glial fibrillary acidic protein (GFAP) Stain and then image.
[0156] The brain tissue sample prepared in Example 1 with a diameter of 10 mm and a thickness of 1 mm was inserted into the holder body of the sample chamber in the device prepared in Example 2, and inserted into the biological sample fixing part to be fixed, and then the electrode part and the sample Each space of the chamber was filled with borate buffer (50 mM LiOH, 25 mM boric acid). An antibody targeting GFAP (primary antibody, Abcam, UK) and an antibody targeting the Fc part of the primary antibody labeled with a fluorescent material (secondary anti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com