A kind of preparation method of 2,5-furandicarboxylic acid under mild conditions
A technology of furandicarboxylic acid and furanaldehyde, applied in the direction of organic chemistry and the like, can solve the problems of less than 95% FDCA yield, increased ineffective loss of 5-HMF, poor catalyst reusability, etc., and achieves reduction of ineffective degradation and The probability of occurrence of side reactions, the effect of improving effective utilization and reducing ineffective decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] An aspect of the embodiments of the present invention provides a method for preparing 2,5-furandicarboxylic acid under mild conditions, comprising:
[0019] reacting the mixed reaction system comprising 5-hydroxymethylfurfural and / or 5-hydroxymethylfurfural derivatives, hydrogen peroxide, catalyst, hydrogen peroxide stabilizer, alkali and water at 0-80°C for 1-24h, 2,5-furandicarboxylic acid is prepared, wherein the pH value of the mixed reaction system is controlled to be 7-13.
[0020] In some more specific embodiments, the preparation method comprises: mixing 5-hydroxymethylfurfural and / or 5-hydroxymethylfurfural derivatives, a catalyst, a hydrogen peroxide stabilizer with water, and then adding the Slowly add hydrogen peroxide dropwise for reaction, and use a base to control the pH of the mixed reaction system to be 8-12 to prepare the 2,5-furandicarboxylic acid.
[0021] Further, the final molar ratio of hydrogen peroxide to 5-hydroxymethylfurfural and / or 5-hydrox...
Embodiment 1
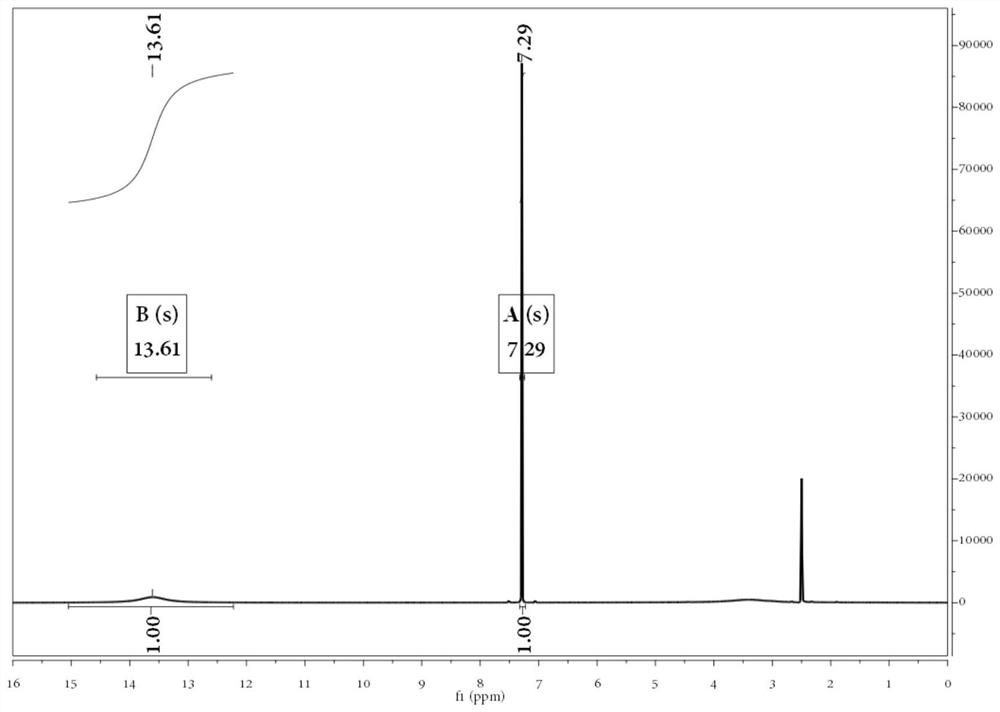
[0056] Weigh 5.0g 5-HMF, 0.01g CuCl 2 , 0.5g EDTA, 0.1g tetramethylpiperidine nitrogen oxide, dissolved in 50g water, then adjusted the pH value of the solution to 10.1 with sodium carbonate, added the solution to a water bath at 40°C and heated, and after the temperature stabilized, within 3 hours Add 14.2g of 30wt% hydrogen peroxide to the solution at a uniform speed, and then continue to keep warm and stir for 2 hours. During the reaction, add sodium carbonate in time to adjust the pH value to about 10.0. FDCA concentration is 88g / kg in sampling measurement solution after reaction finishes, and FDCA productive rate is 99.3%, and FDCA selectivity is 99.6%, and the effective utilization rate of hydrogen peroxide is 95.1%, and 2,5-furandicarboxylic acid (FDCA) 1 H-NMR spectrum such as figure 1 shown.
Embodiment 2
[0058] Weigh 10.0g 5-HMF, 0.03g ferric nitrate, 0.5g diethylenetriaminepentamethylenephosphonic acid, 0.1g 4-acetamido-2,2,6,6-tetramethylpiperidine nitrogen oxide, Use 50g of water to dissolve, then use potassium carbonate to adjust the pH value of the solution to 9.2, add the solution to a 50°C water bath and heat it, after the temperature stabilizes, add 28.0g of 30% hydrogen peroxide to the solution at a uniform speed within 4 hours, and then continue Insulated and stirred for 1 hour, potassium carbonate was added in time during the reaction to adjust the pH value to about 9.0. After the reaction, the concentration of FDCA in the sample measurement solution was 136g / kg, the yield of FDCA was 98.8%, the selectivity of FDCA was 99.5%, and the effective utilization rate of hydrogen peroxide was 96.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com