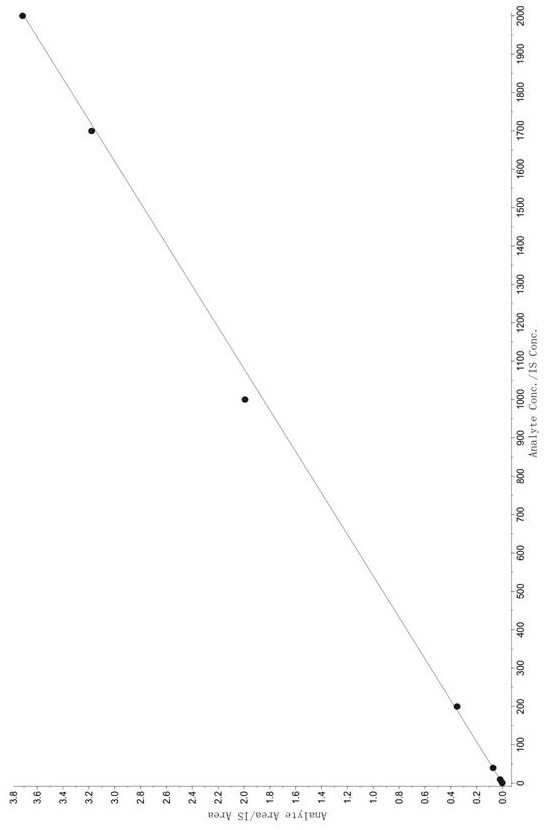
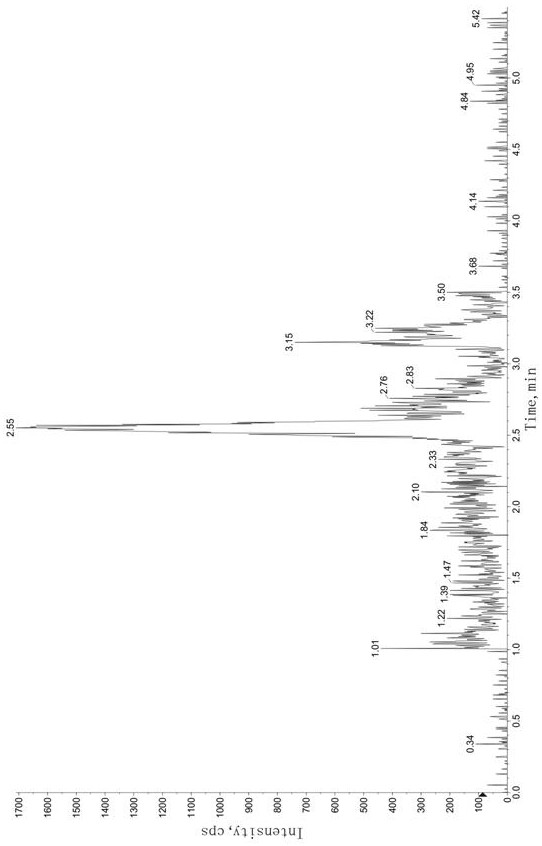
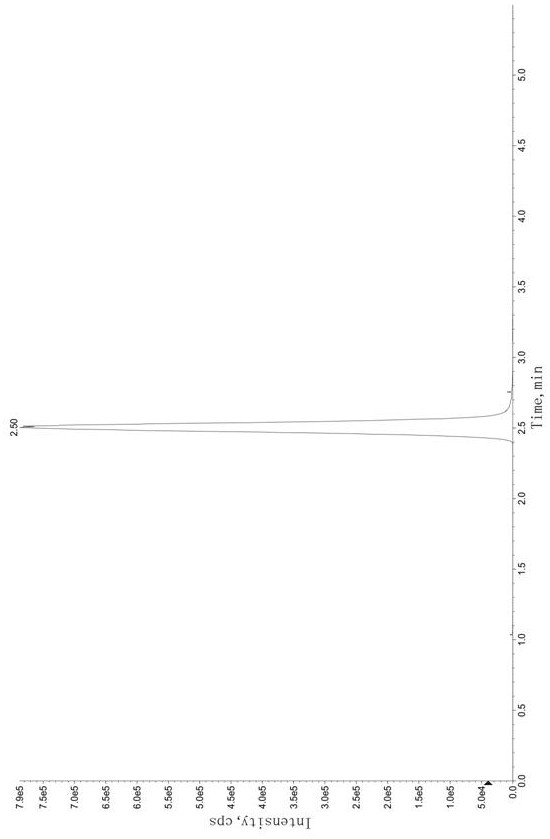
Method for Determination of Tandospirone Concentration in Human Plasma
A technology for tandospirenone and human plasma, which is applied in the field of medical testing, can solve the problems that the lower limit of detection cannot meet the content determination, low-precision quantitative detection, and the determination cannot be realized, etc., and achieves the effects of high accuracy, simple operation and stable baseline.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] 1. Solution preparation
[0093] Mobile phase A (5mM ammonium acetate aqueous solution): Weigh 770.8mg of ammonium acetate and dissolve it in 2L of ultrapure water, ultrasonically degas, and mix well.
[0094] Mobile phase B (acetonitrile): Measure 2L of acetonitrile into a brown solvent bottle.
[0095] Diluted solution (50% acetonitrile in water): transfer 100mL of acetonitrile and 100mL of ultrapure water to an appropriate solvent bottle, and mix well.
[0096] Preparation of needle washing solution (50% methanol aqueous solution): Measure 500mL of methanol and 500mL of ultrapure water, transfer them to an appropriate solvent bottle, and mix well.
[0097] 2. Standard solution preparation:
[0098] Preparation of standard reference substance stock solution
[0099] Weigh a certain amount of tandospirone reference substance, record the weight, put it in a self-made cup-shaped aluminum foil paper, put the aluminum foil paper in a 20mL brown wide-mouth glass bottle, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com