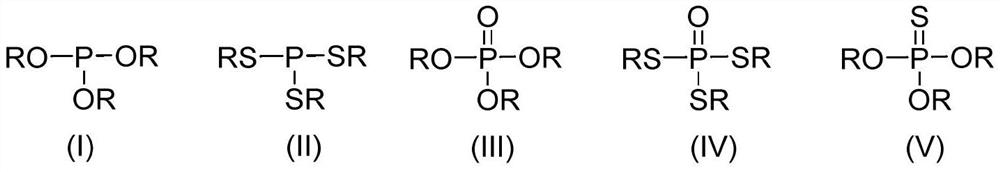
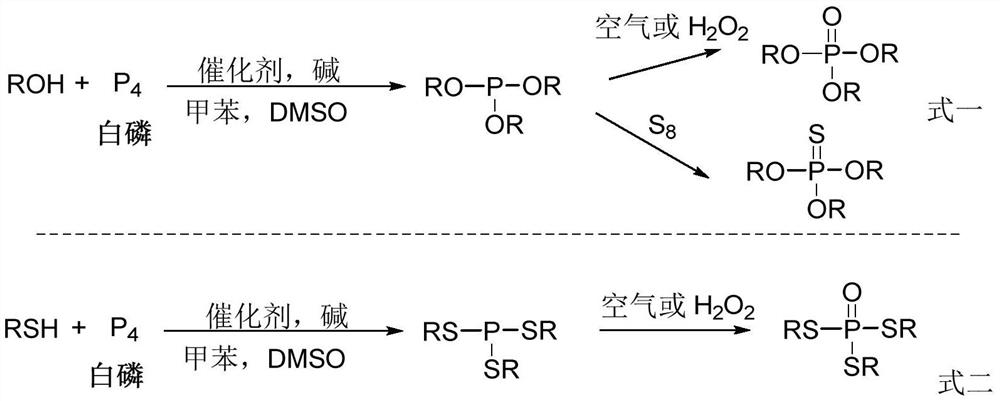
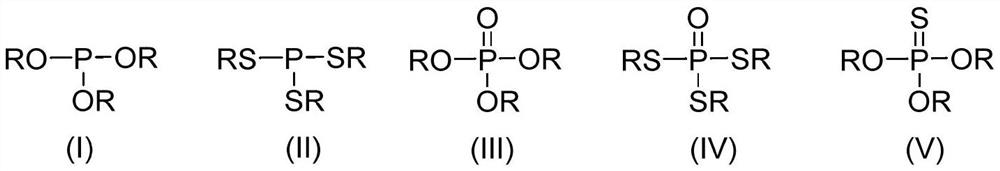
Method for preparing phosphate ester derivatives from white phosphorus
A technology of phosphate esters and derivatives, applied in the chemical industry, can solve the problems of poor atom economy, unfriendly environment, violent reaction, etc., and achieve the effects of low reaction cost, no white phosphorus residue, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of tri-p-methoxyphenyl phosphite
[0028]
[0029] In the 10mL reaction bottle, first add K 3 PO 4 (0.045mmol, 9.6mg), diphenyl diselenide (0.045mmol, 14.1mg), white phosphorus toluene solution (white phosphorus 5.6mg, toluene 0.5mL), p-methoxyphenol (0.9mmol, 112.0mg), di Methyl sulfoxide (0.5 mL) was heated up to 60° C. under argon atmosphere, and reacted for about 4 hours. The reaction was detected by phosphospectrum and the reaction was stopped. The reaction solution was cooled to room temperature, and the reaction solution was separated by silica gel column chromatography (eluent: petroleum ether: ethyl acetate = 10:1, v / v) to obtain 61.3 mg of the product tri-p-methoxyphenyl phosphite. rate of 85%. 1 H NMR (400MHz, CDCl 3 ):δ7.07(d, J=7.4Hz, 6H), 6.86(d, J=7.5Hz, 6H), 3.80(s, 9H). 13 C NMR (100MHz, CDCl 3 ): δ156.2, 145.1 (d, J=3.3Hz), 121.7 (d, J=6.3Hz), 114.7, 55.6. 31 P NMR (162MHz, CDCl 3 ):δ123.83.
Embodiment 2~7
[0030] Embodiment 2~7: the preparation of tri-p-methoxyphenyl phosphite
[0031]
[0032] In addition to the change of the type of alkali, the amount of other substances and the reaction conditions have no change, the results are shown in Table 1:
[0033] Table 1
[0034] Example alkali Yield Example alkali Yield 2 KOH 76% 5 Na 2 CO 3
Embodiment 8
[0035] Embodiment 8: the preparation of triphenyl phosphite
[0036]
[0037] In the 10mL reaction bottle, first add K 3 PO 4 (0.045mmol, 9.6mg), diphenyldiselenide (0.045mmol, 14.1mg), white phosphorus toluene solution (white phosphorus 5.6mg, toluene 0.5mL), phenol (1.1mmol, 101.0mg), dimethyl sulfoxide ( 0.5mL), under the condition of argon, the temperature was raised to 60°C, and the reaction was carried out for about 6 hours. When the reaction was detected by phosphospectrum, the reaction was stopped. The reaction solution was cooled to room temperature, and the reaction solution was separated by silica gel column chromatography (eluent: petroleum ether: ethyl acetate = 10:1, v / v) to obtain 49.1 mg of the product triphenyl phosphite, with a yield of 88%. 1 H NMR (400MHz, CDCl 3 ):δ7.45-7.30(m,6H),7.25-7.21(m,3H),7.21-7.13(m,6H). 13 C NMR (100MHz, CDCl 3 ): δ151.6(d, J=2.7Hz), 129.7, 124.3, 120.8(d, J=6.9Hz). 31 P NMR (162MHz, CDCl 3 ):δ127.84.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com