HB-NC4 recombinant protein and preparation method and application thereof
A technology of HB-NC4 and recombinant protein, which is applied in the field of HB-NC4 recombinant protein and its preparation, can solve the problems that have not existed yet, and achieve the effect of long residence time and strong targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
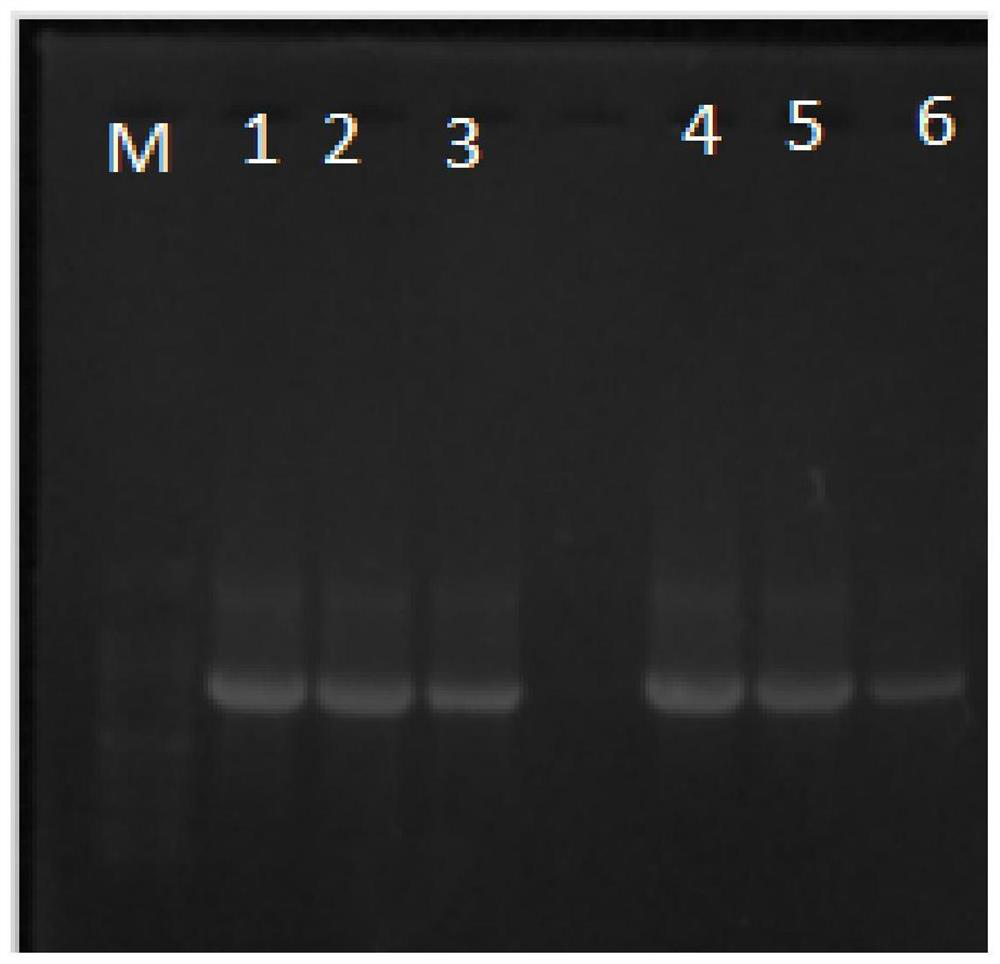
[0118] The preparation method of HB-NC4 recombinant protein of the present invention, the steps are as follows:
[0119] (1) Construction of fusion gene: prepare the HB-NC4 fusion gene containing the target gene encoding NC4 and the target gene encoding HB, and amplify;
[0120] (2) Double digestion of the empty plasmid pET28a: use XhoⅠ and BamHI to perform double digestion of the Escherichia coli expression plasmid pET28a, and recover the fragments;
[0121] (3) Ligation of the fusion gene and the plasmid: Add 31.92ng of the recovered product from PCR and 107.38ng of the recovered product from the digestion of the empty plasmid into the reaction system of the ClonExpress@Ⅱ kit, react at 37°C for 30min, and the ligated product is used for the next step of transformation ;
[0122] (4) Transformation of recombinant plasmids: add 4 μL of cooled reaction solution to 100 μL of LDH5α competent cells and mix well, bathe in ice for 30 minutes, heat shock at 42°C for 90 seconds, bath...
experiment example 1
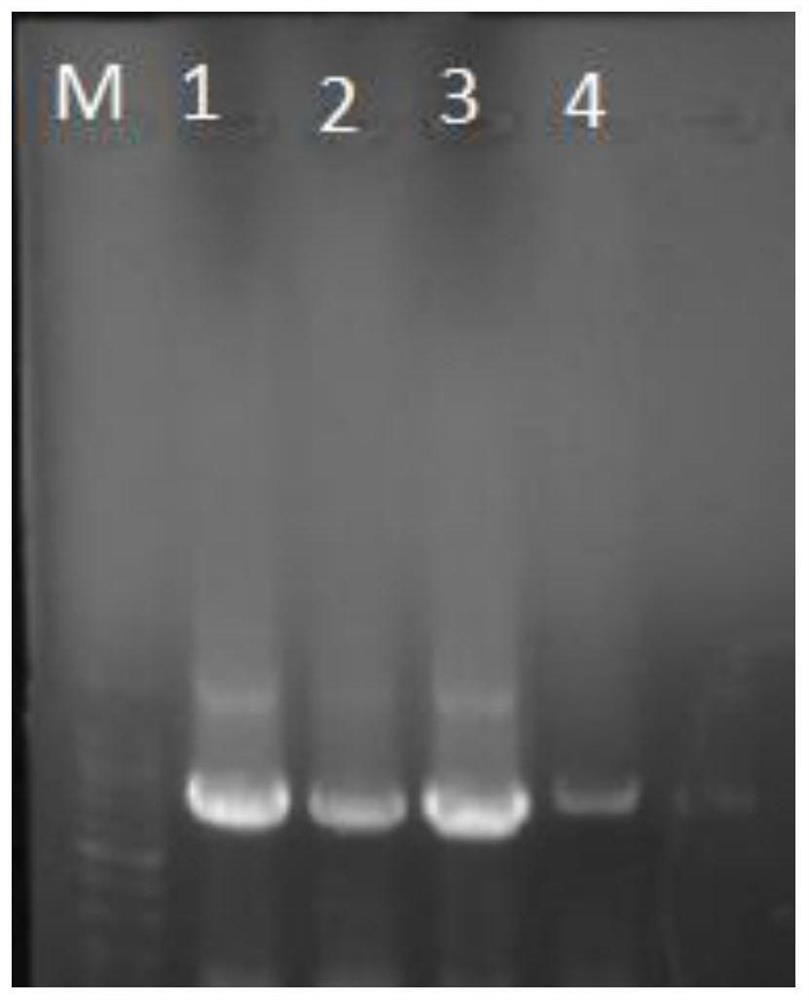
[0129] Preparation of HB-NC4 fusion protein:
[0130] 1. The construction of fusion gene: adopt the method for PCR to obtain fusion gene with NC4 as template, and reclaim.
[0131] 2. Double digestion of the empty plasmid pET28a: XhoⅠ and BamHI were used to perform double digestion of the Escherichia coli expression plasmid pET28a, and the fragments were recovered.
[0132] 3. Ligation of the fusion gene and the plasmid: Add 31.92ng of the recovered product from PCR and 107.38ng of the recovered product from the digestion of the empty plasmid into the reaction system of the ClonExpress@Ⅱ kit, react at 37°C for 30min, and the ligated product is used for the next step of transformation.
[0133] 4. Transformation of recombinant plasmids: Add 4 μL of cooled reaction solution to 100 μL of LDH5α competent cells and mix well, ice-bath for 30 minutes, heat shock at 42°C for 90 seconds, ice-water bath for 2 minutes, add 900 μL of LB liquid medium, and incubate at 37°C Fully recover f...
experiment example 2
[0140] Determination of anti-complement activity of fusion protein:
[0141] Investigate the anti-complement activation activity of HB-NC4: with NC4 as a control (here, the preparation method of the protein as a control is the same as that of HB-NC4), and investigate the anti-complement activation activity of purified HB-NC4 by hemolysis method. The specific implementation steps are as follows :
[0142] First test the protein samples NC4 and HB-NC4 to inhibit the activity of the alternative complement pathway, take the rabbit red blood cell suspension, and use Mg 2+ - Wash with EGTA buffer three times until the supernatant is transparent and colorless. Reuse Mg 2+ - EGTA buffer dilutes the rabbit erythrocyte suspension to a certain concentration. The method for determining the release concentration is as follows: take 10ul of rabbit erythrocyte suspension, add 90ul of deionized water, measure the absorbance at 405nm, repeat the above operation until the absorbance is adjus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com