Glutamate dehydrogenase mutant for producing L-glufosinate-ammonium and L-glufosinate-ammonium production method
A technology of glutamate dehydrogenase and production method, applied in the preparation of L-glufosinate-ammonium, in the field of glutamate dehydrogenase mutants, to achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: microbial cultivation and enzyme activity assay
[0046] 1. Microbial culture
[0047] (1) Composition of LB liquid medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then adjusted to volume, sterilized at 121°C for 20min, ready for use. LB liquid solid medium composition (plate): add agar powder 20g / L on the basis of LB liquid medium, sterilize at 121°C for 20min, cool to 50-60°C, add 50μg / mL kanamycin (Kan), Import into a Petri dish, cool and solidify before use.
[0048] The genetically engineered bacteria E.coli BL21(DE3) was inoculated into 5 mL LB liquid medium containing 50 μg / mL kanamycin, and cultured with shaking at 37°C for 12 hours. Transfer to 500mL fresh LB liquid medium also containing 50μg / mL Kan, shake culture at 37°C until OD 600 When it reaches about 0.8, add isopropylthiogalactoside (IPTG) to a concentration of 0.3mM, and induce culture at 28°C for 20h. After the cultivation, the culture solution ...
Embodiment 2
[0058] Embodiment 2: the construction of genetically engineered bacteria
[0059] 1. Construction of genetically engineered bacteria expressing the original glutamate dehydrogenase
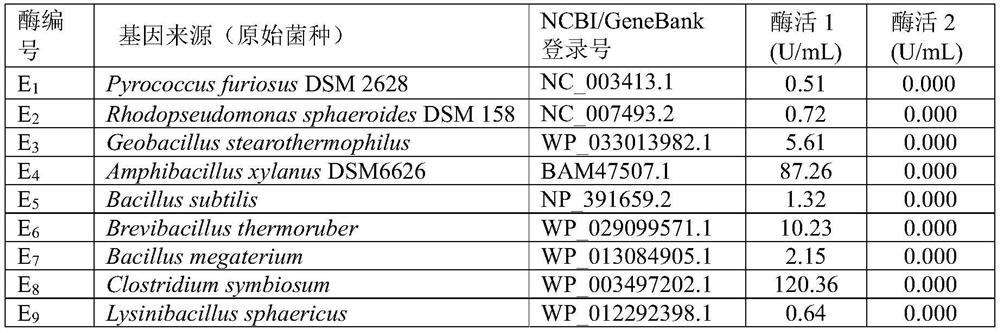
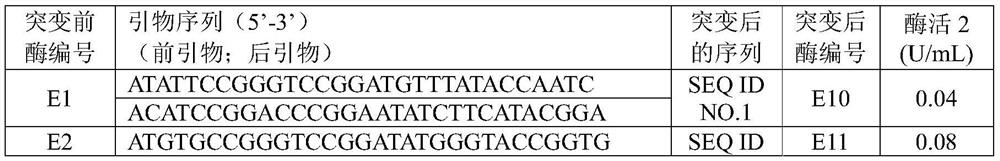
[0060] By comparing the literature data, the original gene sequence of the glutamate dehydrogenase that has high catalytic activity to the natural substrate α-ketoglutarate (the product is L-glutamic acid) and can use NADH as a coenzyme is obtained, such as Table 1 shows.
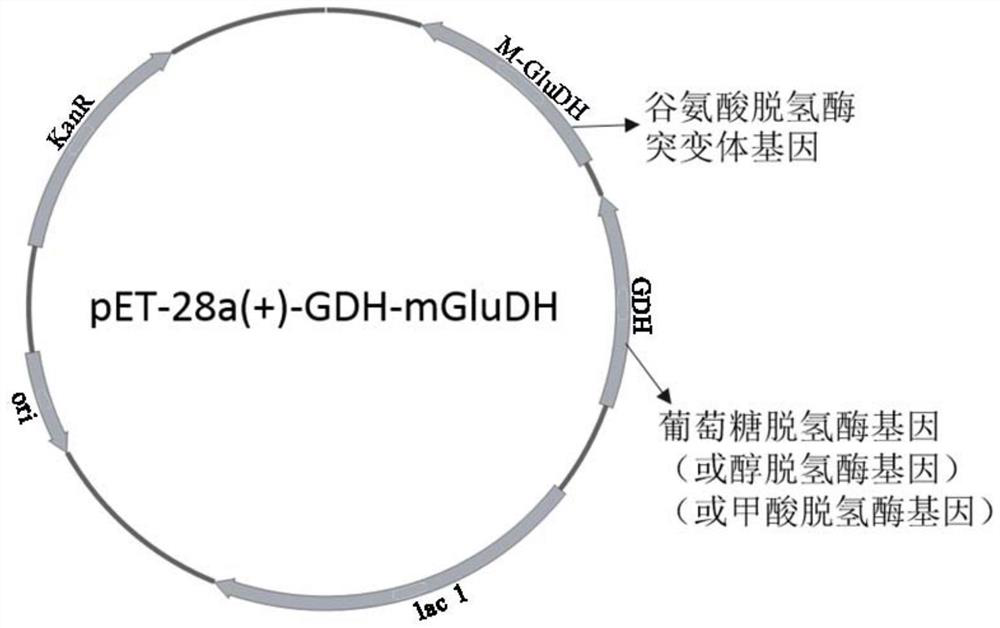
[0061] By querying the gene database NCBI, the gene sequence of the above-mentioned original glutamate dehydrogenase was obtained, sent to Shenggong Bioengineering (Shanghai) Co., Ltd. for whole gene synthesis, and cloned into the recombinant expression plasmid pET-28a(+), inserted into The enzyme cutting sites are BamH I and Xho I. After the recombinant plasmid was verified to be correct by sequencing, it was transferred into the expression host Escherichia coli E.coli BL21 (DE3) for the expression of the original glutamate ...
Embodiment 3
[0081] Example 3: Preparation of L-glufosinate-ammonium using strains co-expressing E10 and E21
[0082] The genetic engineering that co-expresses E10 and E21 is selected, and the cells are collected after being cultured by microorganisms according to the operation in Example 1. The collected bacterial cells were ultrasonically disrupted and centrifuged to remove the precipitate to obtain a crude enzyme solution. Put a solution containing about 200mM PPO and about 250mM glucose in a magnetically stirred reactor, adjust the pH of the solution to 7.5 with 30% ammonia water, and then add NAD + 0.001mM; then add a crude enzyme solution equivalent to a wet cell concentration of 20g / L, control the reaction temperature in a water bath to 20°C, start stirring, use ammonia water to control pH = 7.5, and react for 24 hours. The formation concentration of phosphine was 197.9mM, and the ee value of the detected product glufosinate-ammonium was 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com