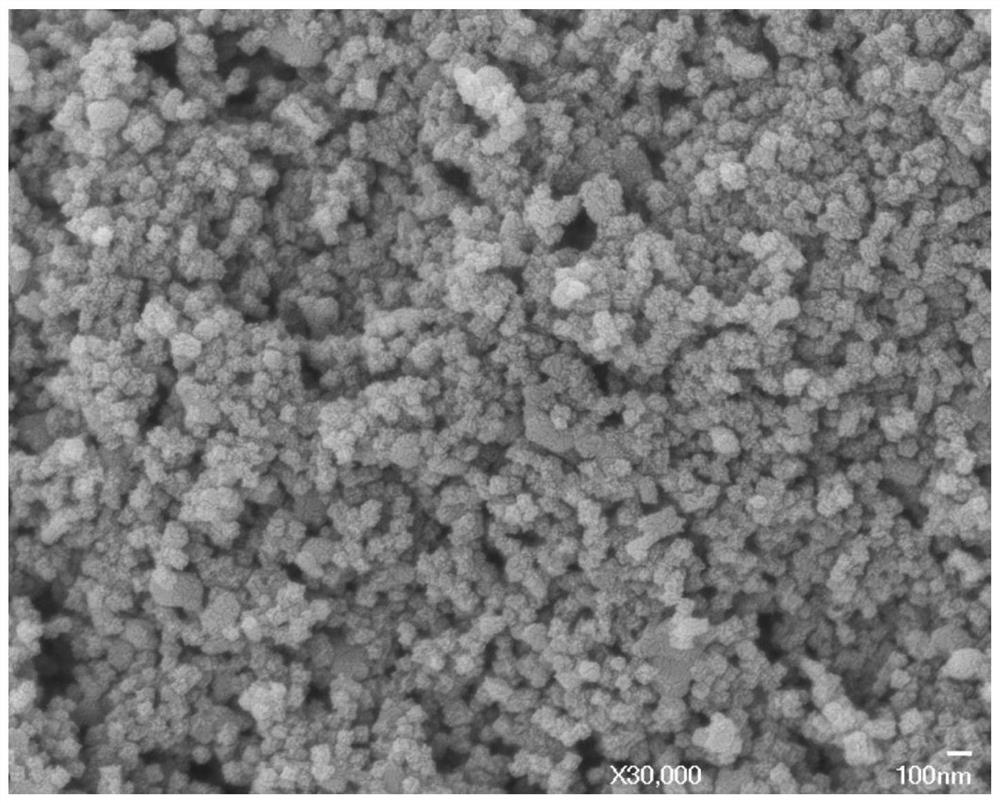
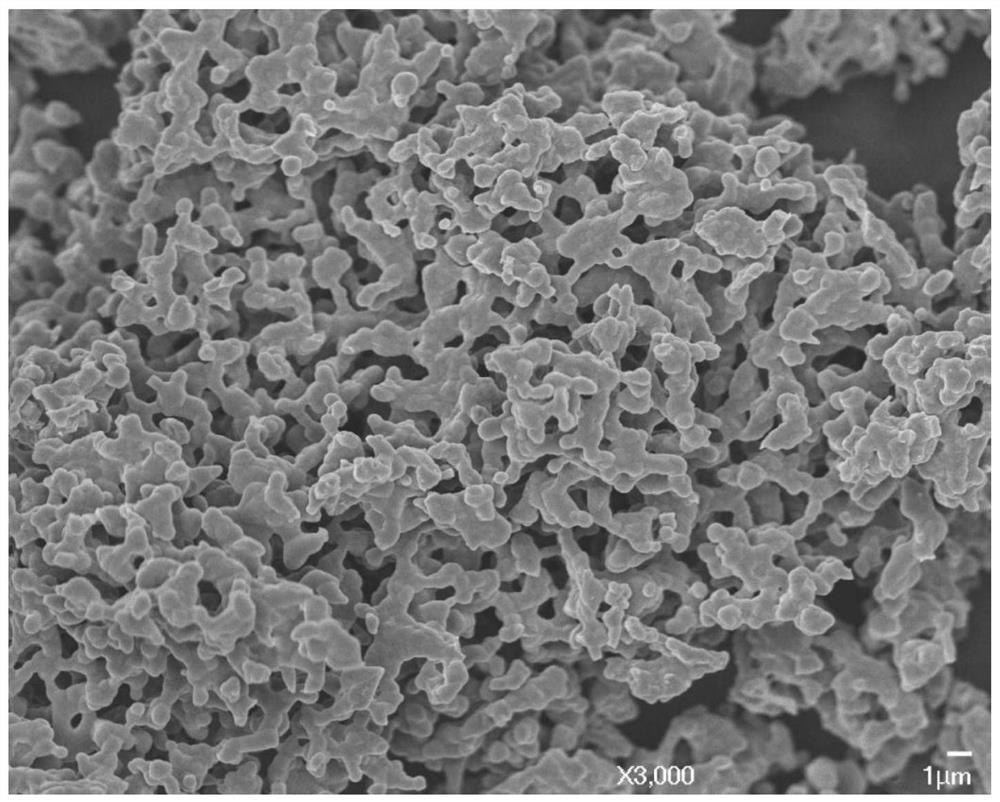
Method for preparing boron carbide powder by adopting organic carbon source
A technology of boron carbide powder and organic carbon, which is applied in the field of preparing boron carbide powder, can solve the problems of high cost of sintering densification, low mechanical properties, low fracture toughness, etc., and achieve full reaction, high performance, and reduced dosage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
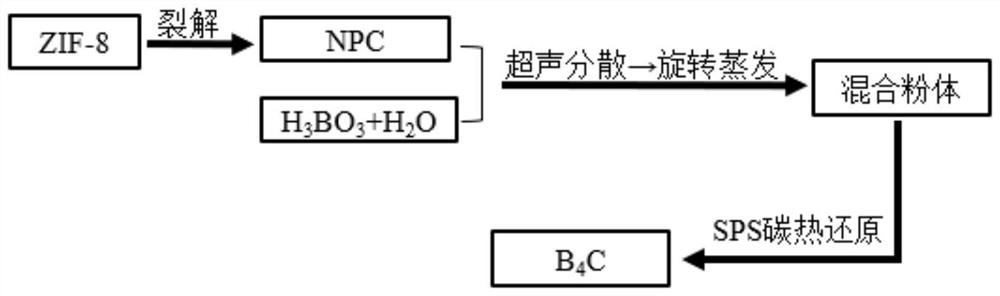
[0038] as per figure 1 The shown flow process adopts the method for preparing boron carbide powder from an organic carbon source, comprising the following steps:
[0039] (1) In two containers, dissolve zinc nitrate hexahydrate and dimethylimidazole in solvent methanol respectively; add magnets to each container, use a magnetic stirrer with heating function, and heat the solutions to 25°C respectively, and continue Magnetic stirring for 1h;
[0040] The molar ratio of zinc nitrate hexahydrate, dimethylimidazole and methanol is 1:2.36:727.
[0041] (2) Pour the solution of zinc nitrate hexahydrate into the dimethylimidazole solution, mix and let stand for 3h;
[0042] (3) Remove the supernatant of the reactant, and obtain the white precipitate of ZIF-8 by centrifugal separation, the centrifugation parameters are 10000rpm, 5min; the white precipitate is washed with methanol, and the methanol is washed three times, and the process of each time is: in the white precipitate Meth...
Embodiment 2
[0049] as per figure 1 The shown flow process adopts the method for preparing boron carbide powder from an organic carbon source, comprising the following steps:
[0050] (1) In two containers, respectively dissolve zinc nitrate hexahydrate and dimethylimidazole in solvent methanol; add magnets to each container, use a magnetic stirrer with a heating function, and heat the solutions to 40°C respectively for continuous Magnetic stirring for 1h;
[0051] The molar ratio of zinc nitrate hexahydrate, dimethylimidazole and methanol is 1:3.36:727.
[0052] (2) Pour the solution of zinc nitrate hexahydrate into the dimethylimidazole solution, mix and let stand for 10h;
[0053] (3) Remove the supernatant of the reactant, and obtain the white precipitate of ZIF-8 by centrifugal separation, the centrifugation parameters are 10000rpm, 5min; the white precipitate is washed with methanol, and the methanol is washed three times, and the process of each time is: in the white precipitate ...
Embodiment 3
[0060] as per figure 1 The shown flow process adopts the method for preparing boron carbide powder from an organic carbon source, comprising the following steps:
[0061] (1) In two containers, respectively dissolve zinc nitrate hexahydrate and dimethylimidazole in solvent methanol; add magnets to each container, use a magnetic stirrer with heating function, and heat the solutions to 50°C respectively for Magnetic stirring for 1.3h;
[0062] The molar ratio of zinc nitrate hexahydrate, dimethylimidazole and methanol is 1:4.48:727.
[0063] (2) Pour the solution of zinc nitrate hexahydrate into the dimethylimidazole solution, mix and let stand for 15h;
[0064] (3) Remove the supernatant of the reactant, and obtain the white precipitate of ZIF-8 by centrifugal separation, the centrifugation parameters are 10000rpm, 5min; the white precipitate is washed with methanol, and the methanol is washed three times, and the process of each time is: in the white precipitate Methanol wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com