Method for preparing high-purity dilinoyl phosphatidylcholine through combination of enzymatic modification and reversed-phase column chromatography separation and product of high-purity dilinoyl phosphatidylcholine
A technology of dilinoleoylphosphatidylcholine and phosphatidylcholine, which is applied in the field of preparation of high-purity dilinoleoylphosphatidylcholine by enzymatic modification combined with reversed-phase column chromatography, can solve complex production process and product Low purity, long reaction time and other problems, to achieve the effect of good repeatability, simple and controllable operation, and low equipment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This example provides a method for preparing high-purity dilinoleoylphosphatidylcholine and its product through enzymatic modification combined with reversed-phase column chromatographic separation.
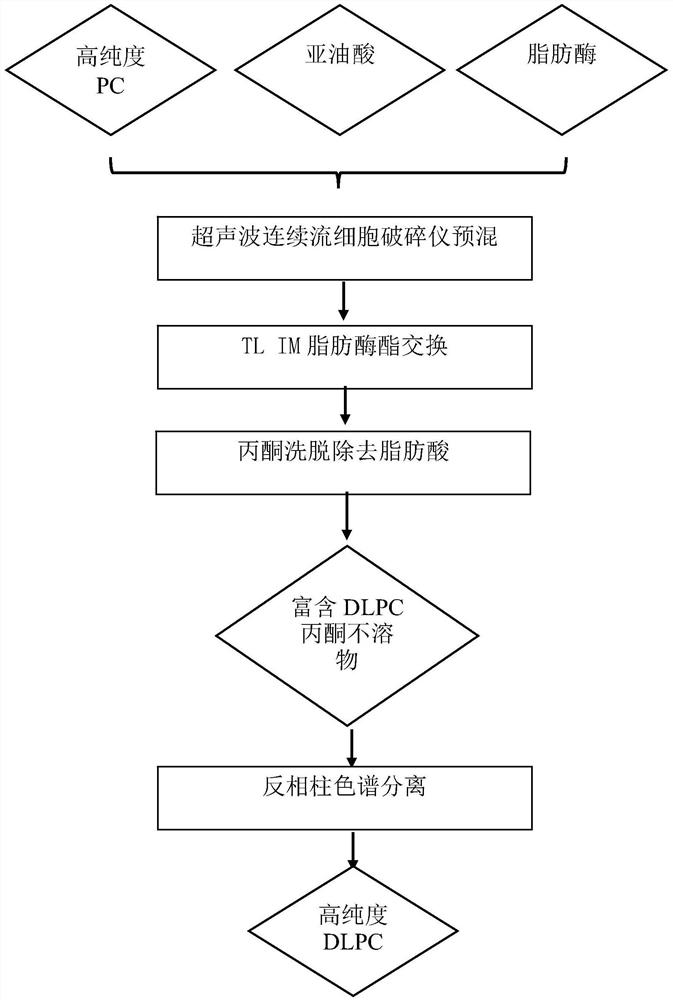
[0038] figure 1 Show the flow chart of the preparation DLPC of an embodiment of the present invention, see figure 1 , add 3.5% (moisture: high-purity phosphatidylcholine + linoleic acid, m / m) to the mixed solution of 2g high-purity phosphatidylcholine, 4g linoleic acid and 10mL n-hexane, add 5% TL IM fat Enzyme (lipase: high-purity phosphatidylcholine + linoleic acid, m / m), the above mixture was premixed under the ultrasonic continuous flow cell disruptor, the premixing power was 200W, ultrasonic 5s / intermittent 5s, repeated 5 times , total ultrasound 45s. The above mixture was stirred and reacted at 50° C. for 1 h to obtain a modified mixture of phosphatidylcholine and fatty acid. After the phosphatidylcholine mixture was dried at low temperature to remove n-hexane, it...
Embodiment 2
[0041] This example provides a method for preparing high-purity dilinoleoylphosphatidylcholine and its product through enzymatic modification combined with reversed-phase column chromatographic separation.
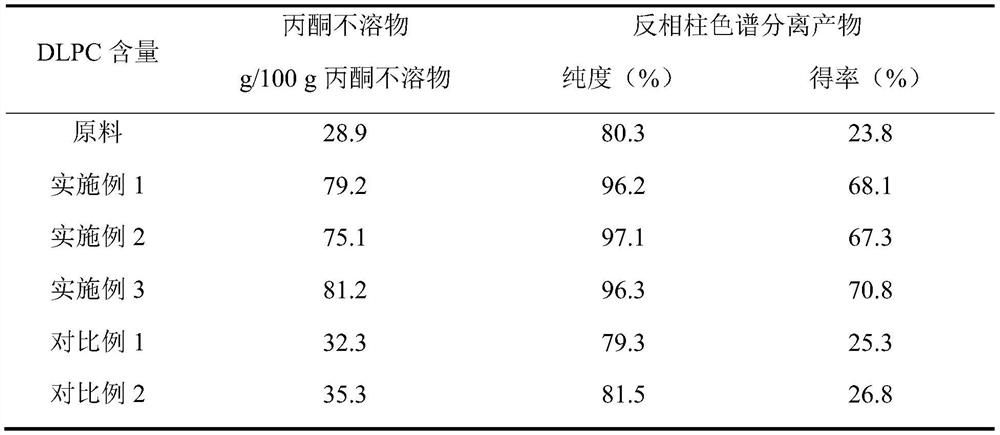
[0042] Add 3.5% (moisture: high-purity phosphatidylcholine + linoleic acid, m / m) to the mixed solution of 2g high-purity phosphatidylcholine, 4g linoleic acid and 10mL n-hexane, add 5% TL IM lipase (Lipase: high-purity phosphatidylcholine+linoleic acid, m / m), the above mixture is premixed under an ultrasonic continuous flow cell disruptor, the premixing power is 500W, ultrasonic 5s / intermittent 5s, repeat 3 times, A total of 15s of ultrasound. The above mixture was stirred and reacted at 50° C. for 1 h to obtain a modified mixture of phosphatidylcholine and fatty acid. After the phosphatidylcholine mixture was dried at low temperature to remove n-hexane, it was eluted three times with acetone, and the acetone insoluble matter was collected, wherein the content of dilinole...
Embodiment 3
[0045] This example provides a method for preparing high-purity dilinoleoylphosphatidylcholine and its product through enzymatic modification combined with reversed-phase column chromatographic separation.
[0046] Add 3.5% (moisture: high-purity phosphatidylcholine + linoleic acid, m / m) to 20g high-purity phosphatidylcholine, 40g linoleic acid and 100mL n-hexane mixed solution, add 5% TL IM lipase (Lipase: high-purity phosphatidylcholine+linoleic acid, m / m), the above mixture is premixed under an ultrasonic continuous flow cell disruptor, the premixing power is 800W, ultrasonic 5s / intermittent 5s, repeat 10 times, A total of 95s of ultrasound. The above mixture was stirred and reacted at 50° C. for 1 h to obtain a modified mixture of phosphatidylcholine and fatty acid. After the phosphatidylcholine mixture was dried at low temperature to remove n-hexane, it was eluted with acetone three times to collect the acetone insoluble matter, wherein the content of dilinoleoylphosphat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com