Ortho-halogenated arylamine compound and synthesis method thereof
A halogenated aryl amine and a synthetic method technology, applied in the field of organic chemical synthesis, can solve problems such as bad by-products, difficult to separate, high reagents, etc., and achieve the effects of good tolerance, excellent yield, and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
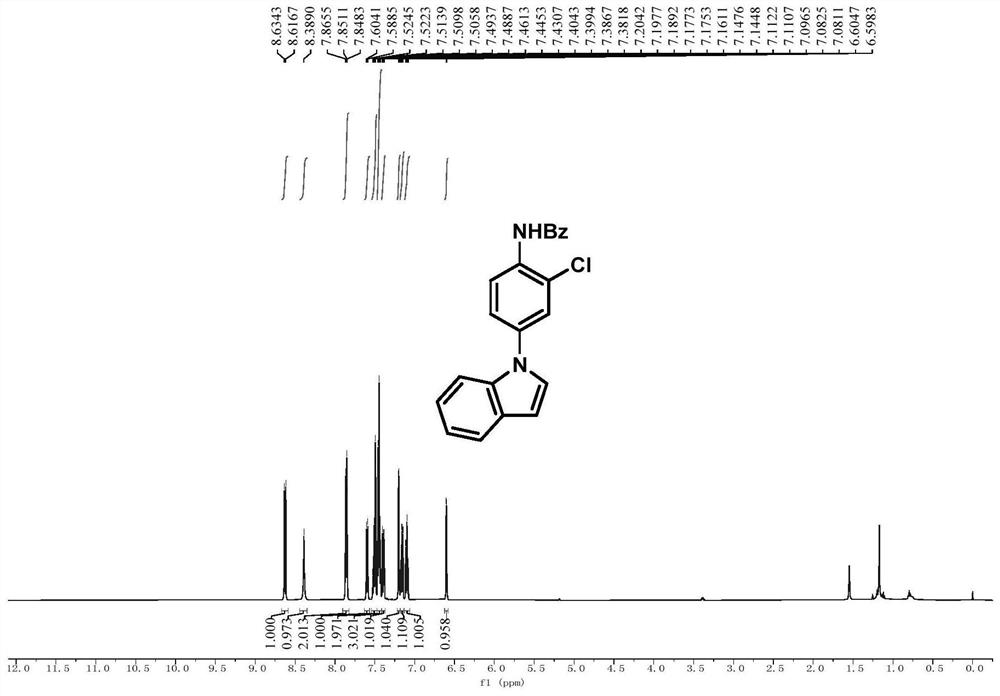
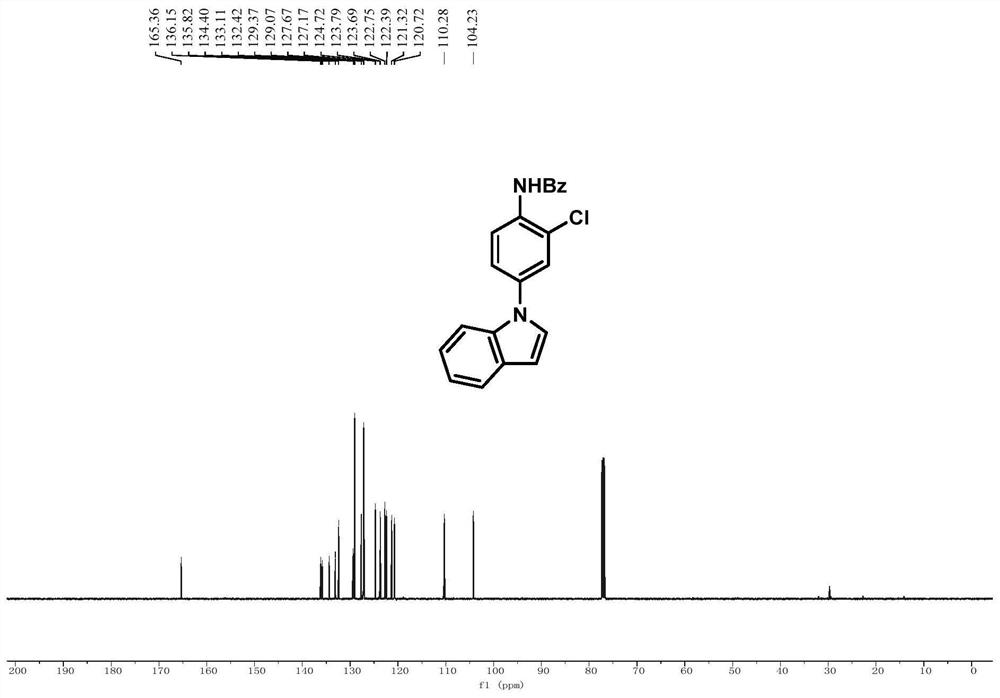
[0081] Embodiment 1, N-(2-chloro-4-(1H-indol-1-yl)phenyl)benzamide
[0082]
[0083] In a 25mL Schlenk tube, add N-(4-(1H-indol-1-yl)phenyl)-N-hydroxybenzamide (0.2mmol, 66mg) and sodium carbonate (0.1mmol), and purify with nitrogen After three times, tetrahydrofuran (1 mL) was added in a nitrogen atmosphere, and after cooling to 0 °C, thionyl chloride (0.24 mmol) was added dropwise while stirring at 0 °C. After the addition was completed, the reaction process was tracked by TLC. After the reaction was completed, the reaction mixture was removed by rotary evaporation to remove the solvent, and the crude product was subjected to column chromatography (eluent: petroleum ether: ethyl acetate = 5:1) to obtain pure white powder N-(2-chloro-4-( 1H-indol-1-yl)phenyl)benzamide, the yield was 66%.
[0084] 1 H NMR (500MHz, CDCl 3 ):δ8.63(d,J=8.8Hz,1H),8.39(s,1H),7.90-7.81(m,2H),7.60(d,J=7.8Hz,1H),7.55-7.47(m, 2H), 7.45(t, J=7.6Hz, 3H), 7.39(dd, J=8.8, 2.4Hz, 1H), 7.20(t, J=3.8Hz...
Embodiment 2
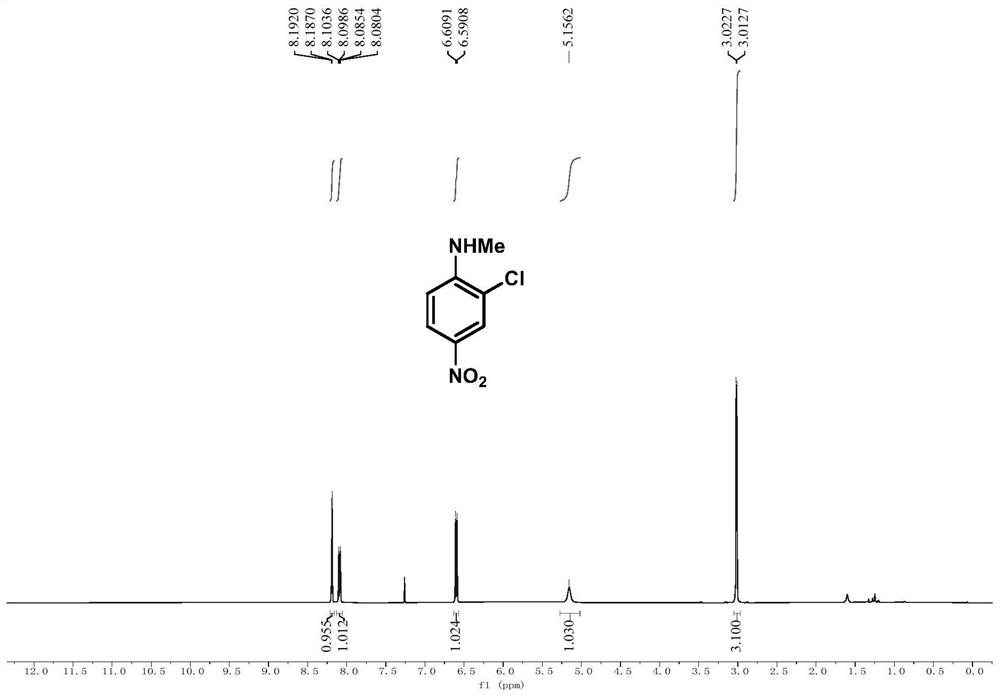
[0086] Embodiment 2, 2-chloro-N-methyl-4-nitroaniline
[0087]
[0088] In a 25mL Schlenk tube, add N-methyl-N-(4-nitrophenyl)hydroxylamine (0.2mmol, 34mg) and sodium carbonate (0.1mmol), after three times of nitrogen pumping, add tetrahydrofuran under nitrogen atmosphere (1mL), after cooling to 0°C, thionyl chloride (0.24mmol) was added dropwise while stirring at 0°C, after the dropwise addition was completed, the reaction process was tracked by TLC, and after the reaction was completed, the reaction mixture was evaporated by rotary evaporation The solvent was removed, and the crude product was subjected to column chromatography (eluent: petroleum ether: ethyl acetate = 5:1) to obtain pure product 2-chloro-N-methyl-4-nitroaniline in the form of colorless oil with a yield of 65 %.
[0089] 1 H NMR (500MHz, CDCl 3):δ8.19(d,J=2.5Hz,1H),8.09(dd,J=9.1,2.5Hz,1H),6.60(d,J=9.1Hz,1H),5.16(s,1H),3.02 (d, J=5.0Hz, 3H).
[0090] 13 C NMR (126MHz, CDCl 3 ): δ149.8, 137.4, 125.3,...
Embodiment 3
[0091] Example 3, N-(2-chloro-6-methoxy-5-(triisopropylsilyl)ethynyl)pyridin-3-yl)benzamide
[0092]
[0093] In a 25 mL Schlenk tube, add N-hydroxy-N-(6-methoxy-5-(triisopropylsilyl)ethynyl)pyridin-3-yl)benzamide (0.2 mmol, 85 mg) and Sodium carbonate (0.1mmol), nitrogen pumped and ventilated three times, tetrahydrofuran (1mL) was added in nitrogen atmosphere, after cooling to 0°C, thionyl chloride (0.24mmol) was added dropwise while stirring at 0°C, and After the completion, the reaction process was tracked by TLC. After the reaction was completed, the reaction mixture was removed from the solvent by rotary evaporation, and the crude product was subjected to column chromatography (eluent: petroleum ether:ethyl acetate=5:1) to obtain pure white powder. Product N-(2-chloro-6-methoxy-5-(triisopropylsilyl)ethynyl)pyridin-3-yl)benzamide, the yield was 95%.
[0094] 1 H NMR (500MHz, CDCl 3 ): δ8.81(s,1H),8.10(s,1H),7.90(d,J=7.6Hz,2H),7.59(t,J=7.3Hz,1H),7.52(t,J=7.6Hz ,2H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com