Preparation method of antibacterial glycopeptide hydrogel
A hydrogel and antibacterial sugar technology, applied in medical science, bandages, etc., can solve the problems that hinder the wide application of antibacterial hydrogels, the degradation cycle of antibacterial peptides is short, and the quaternary ammonium salt cytotoxicity is high, so as to induce autoimmunity, The effect of promoting wound healing and accelerating wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 discloses a method of preparing an antibacterial peptide hydrogel.
[0051] A method for preparing a hydrogel of antibacterial peptide peptide, including the following steps:
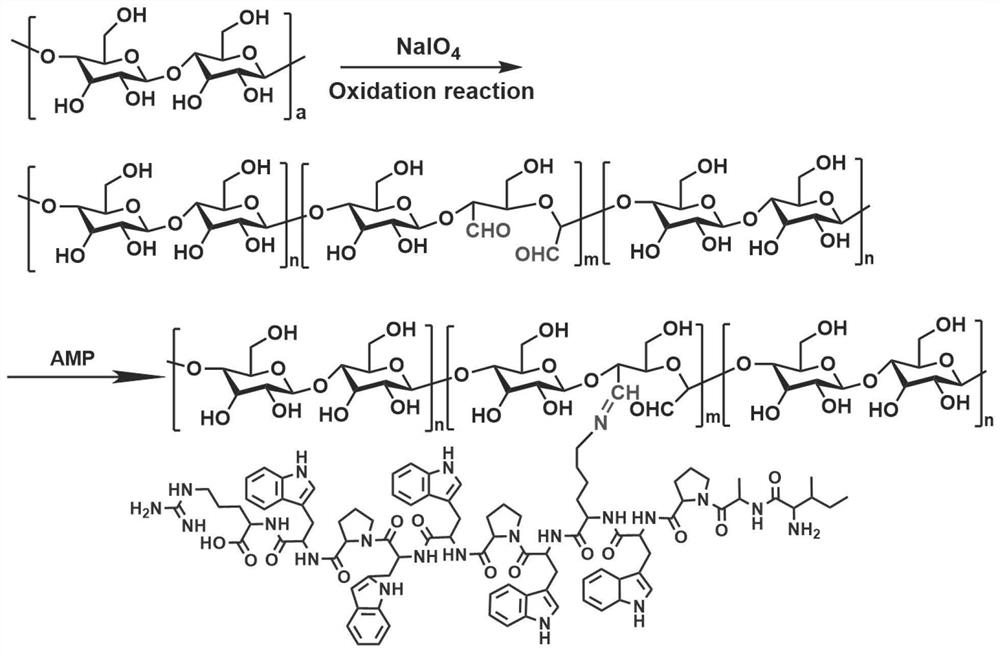
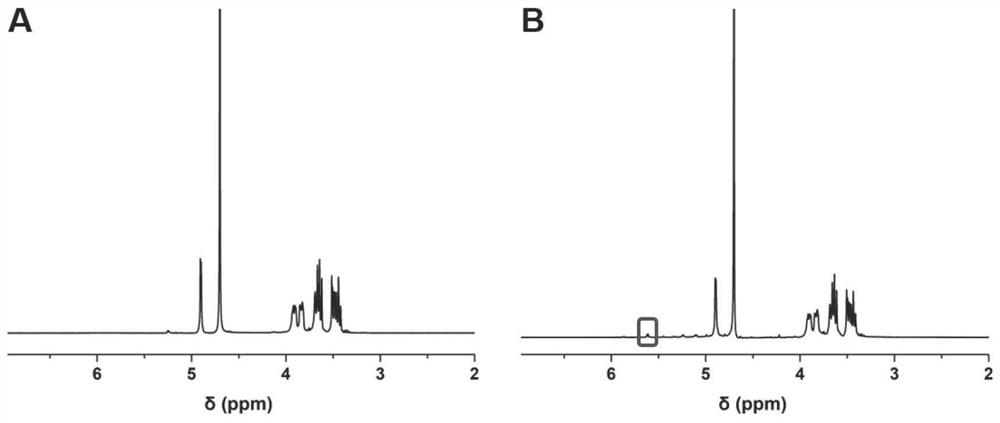
[0052] S1, dissolve the glucosaccharide and the molar ratio of 5: 1 in distilled water according to the molar ratio of 5: 1, reacting under the protected light, and the addition of excess ethylene glycol stopped, dialysis 48h, lyophilized 48 h, to obtain oxidation Gliton (GM-CHO), dissolved GM-CHO with the antibacterial peptide in distilled water at a molar ratio of 5: 1, reacted at room temperature for 48 h, resulting in antibacterial peptide glycan;
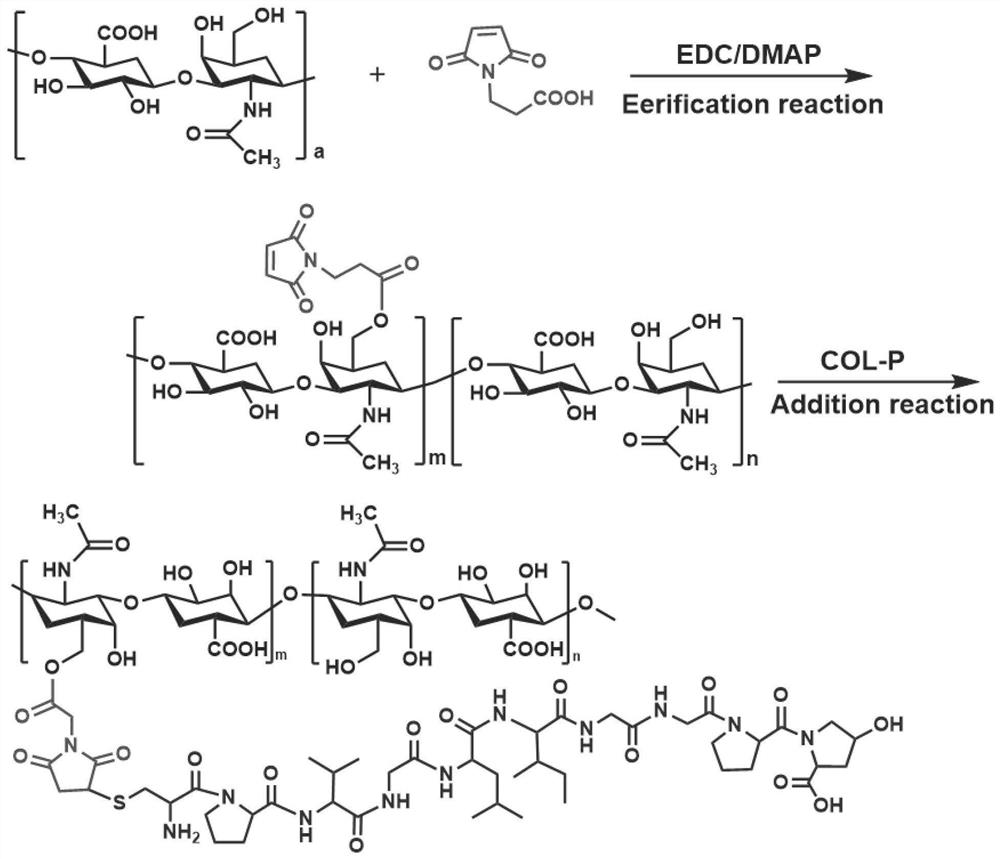
[0053] S2, the hyaluronic acid, 3-maleimide propionic acid is dissolved in distilled water according to the molar ratio of 1: 2, and the catalyst 1- (3-dimethylaminopropyl) 3-ethyl carbon is added thereto. Diimine hydrochloride and 4-dimethylpyridine, at room temperature for 48 h, then lysis, lysis, to obtain esterification hyaluronic acid (...
Embodiment 2
[0056] Example 2 A method of preparing an antimicrobial peptide hydrogel is disclosed.
[0057] A method for preparing a hydrogel of antibacterial peptide peptide, including the following steps:
[0058] S1, dissolve the glucan with high-iodide in distilled water in distilled water according to the molar ratio of 10: 1, and the reaction of excess ethylene glycol is added, 48 h, lyophilized for 48 h, to give GM- CHO, dissolved GM-CHO with the antibacterial peptide in distilled water at a molar ratio of 10: 1, reacted at room temperature for 48 h, then dialyzes lyophilized to give antibacterial peptide glycan;
[0059] S2, the hyaluronic acid, 3-maleimide propionic acid is dissolved in distilled water according to the molar ratio of 1: 1, and the catalyst 1- (3-dimethylaminopropyl) 3-ethyl carbon is added thereto. Dismine hydrochloride and 4-dimethyl amino pyridine react at room temperature for 48 h, to obtain HA-COO, dissolve HA-COO and collagen in distilled water, the molar ratio ...
Embodiment 3
[0062] Example 3 discloses a method of preparing an antibacterial peptide hydrogel.
[0063] A method for preparing a hydrogel of antibacterial peptide peptide, including the following steps:
[0064] S1, dissolve the glucosaccharide with the molar ratio of the sodium of 6: 1 in distilled water in distilled water, and the addition of excess ethylene glycol stopped, dialysis 48h, lyophilized 48 h, resulting in oxidation G. G. Glitrose, the antibacterial peptide was dissolved in distilled water in distilled water at 6: 1, at room temperature to obtain antibacterial peptide glycan;
[0065] S2, the hyaluronic acid, 3-maleimide propionic acid is dissolved in distilled water according to the molar ratio of 1: 1.5, and the catalyst 1- (3-dimethylaminopropyl) 3-ethyl carbon is added thereto. Diimine hydrochloride and 4-dimethylhydridine, at room temperature for 48 h, dialysis 48h, lyophilized 72 h, to obtain HA-COO, dissolve HA-COO and collagen in distilled water, molar ratio is 6: 1 The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com