1, 4-triethoxylbenzyldiammonium sulfate, 1, 3, 5-triethoxylbenzyltriammonium sulfate, synthesis method and application of 1, 4-triethoxylbenzyl diammonium sulfate, and synthesis method and application of 1, 3, 5-triethoxylbenzyl triammonium sulfate
A technology of trihydroxyethyl diammonium and trihydroxyethyl diammonium, applied in 1,4-trihydroxyethyl diammonium sulfate, 1,3,5-trihydroxyethyl diammonium sulfate In the field of salt and synthesis and application, it can solve the problems of desulfurization gypsum quality decline, low environmental impact resistance, gypsum saturated and difficult to digest, and achieve the effects of large-scale production, strong environmental adaptability, and high adsorption saturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
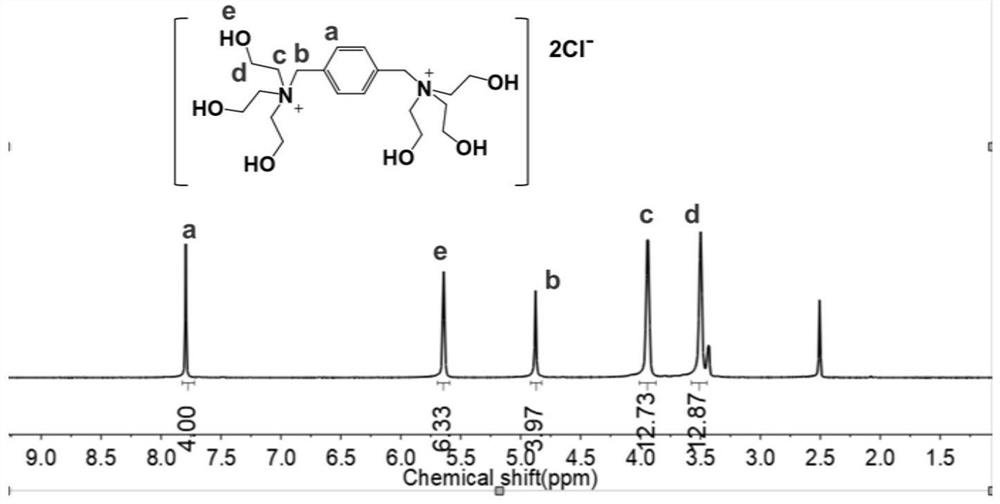
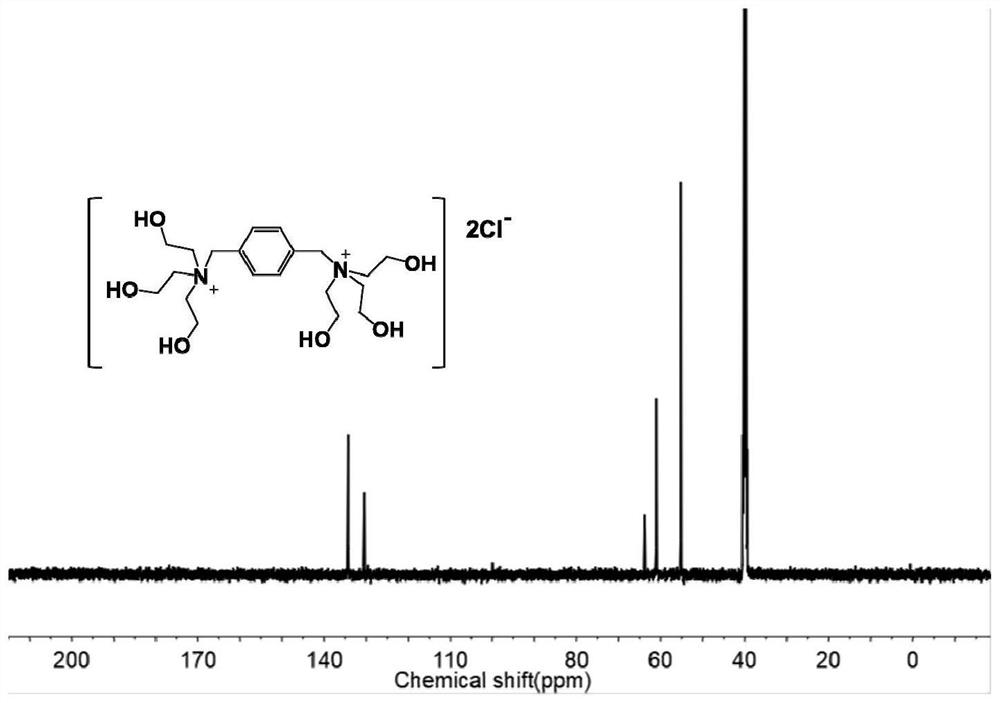
[0054] In this example, 1,4-p-dichlorobenzyl, acetonitrile, triethanolamine, sulfuric acid, etc. are used as main raw materials, and are prepared by chemical synthesis under certain conditions. Specifically include the following main steps:
[0055] (1) Add 1 part of 1,4-p-dichlorobenzyl to 2.5 parts of acetonitrile solution, stir well, and disperse evenly;
[0056] (2) Add 2.2 parts of triethanolamine, stir at the same time and heat the temperature to 80°C for reflux for 8 hours. The reaction equation is:
[0057]
[0058] (3) naturally cool to room temperature, and generate a large amount of 1,4-trihydroxyethyl diammonium chloride white precipitate, obtain filter cake after filtering, filter cake is washed 3 times with acetonitrile solution;
[0059] (4) drying the filter cake for 8 hours under vacuum conditions at 50° C. to obtain 1,4-trihydroxyethylbendiammonium chloride;
[0060] (5) Under stirring conditions, slowly add excess 3% to 6% concentrated sulfuric acid int...
Embodiment 2
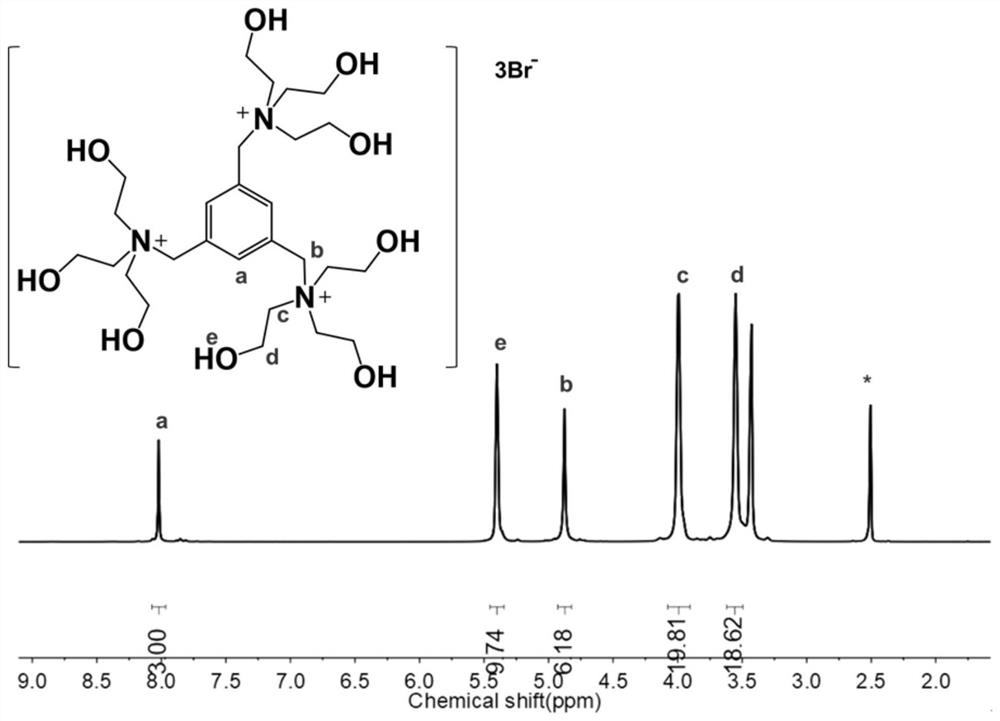
[0068] In this example, 1,3,5-tris(bromomethyl)benzene, acetonitrile, triethanolamine, sulfuric acid, etc. are used as main raw materials, and are prepared by chemical synthesis under certain conditions. Specifically include the following main steps:
[0069] (1) Add 1 part of 1,3,5-tris(bromomethyl)benzene into 2.6 parts of acetonitrile solution, stir well, and disperse evenly;
[0070] (2) Add 3.3 parts of triethanolamine, stir at the same time and heat the temperature to 80°C for reflux for 8 hours. The reaction equation is:
[0071]
[0072] (3) naturally cool to room temperature, and generate a large amount of 1,3,5-trihydroxyethyltriammonium bromide white precipitate, obtain filter cake after filtering, filter cake is washed 3 times with acetonitrile solution;
[0073] (4) drying the filter cake under vacuum conditions at 50°C for 8 hours to obtain 1,3,5-trihydroxyethyltriammonium bromide;
[0074] (5) Under stirring conditions, slowly add excess 3% to 6% concentrat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com