Closed boron group composite nano noble metal catalyst as well as preparation method and application thereof
A noble metal catalyst and noble metal technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, oxidation reaction preparation, chemical instruments and methods, etc. Uniformity and other issues, to achieve the effects of cost controllable, high reuse rate, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
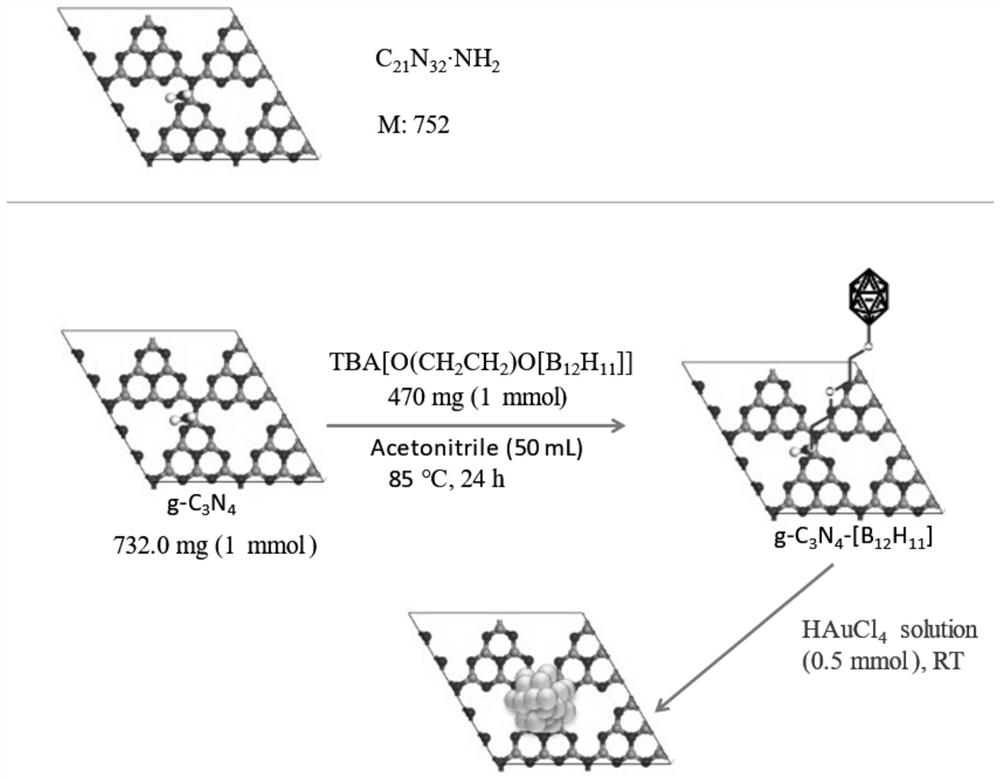
[0043] Preparation of catalyst, synthetic route as attached figure 1 As shown, the steps are as follows:
[0044] (1) 1.0236g g-C 3 N 4 Disperse in 50ml water, add 0.3543g K 2 B 12 h 12 , continuously stirring and reacting at 80°C for 24h;
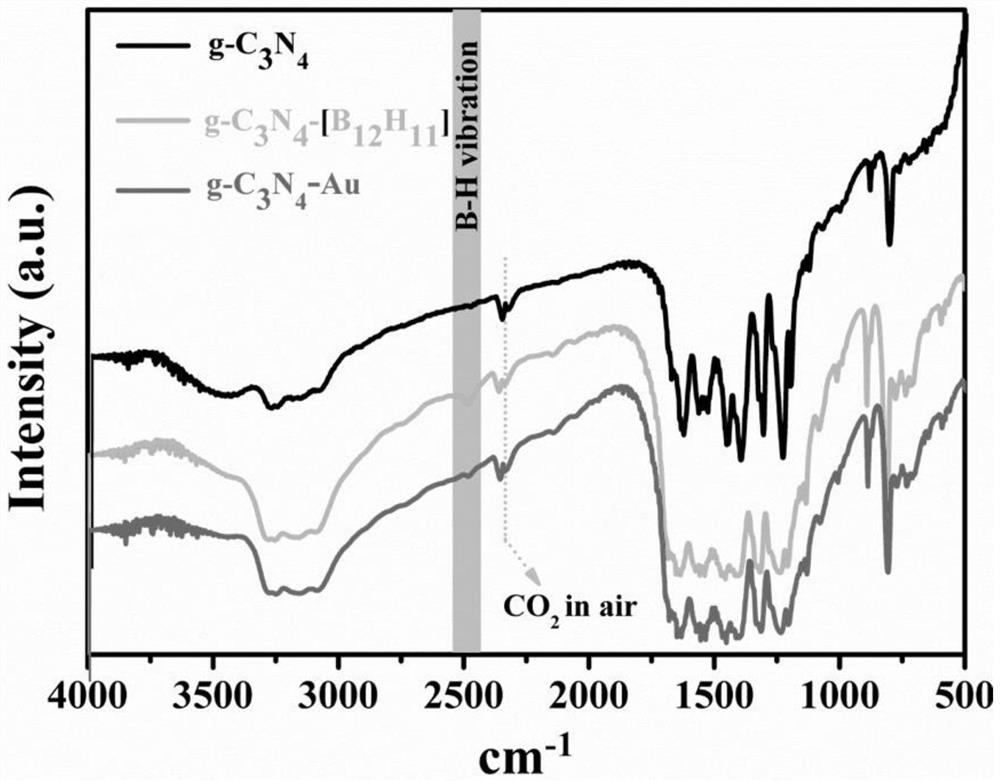
[0045] (2) Filter after 24 hours, wash the filter cake 6 times with water and ethanol successively, and place the washed precipitate at room temperature for 12 hours in vacuum to obtain 1.1024 g of g-C 3 N 4 Base K 2 B 12 h 12 , the material was characterized by Fourier transform infrared spectroscopy (FT-IR) ( figure 2 shown), prove that g-C 3 N 4 Successfully combined with boron clusters;
[0046] (3) 1.0978g g-C 3 N 4 Base K 2 B 12 h 12 Disperse in 20ml of water; quickly add 0.0364g of sodium chloroaurate with a purity of 99%;
[0047] (4) After reacting at 20°C for 10 minutes under a 365nm ultraviolet lamp, filter to obtain a purple precipitate;
[0048] (5) After the precipitate was filtered, the precipitate was w...
Embodiment 2
[0052] Preparation of catalyst, the steps are as follows:
[0053] (1) 1.0246g g-C 3 N 4 Disperse in 50ml water, add 0.6884g Na 2 B 12 h 12 , continuously stirring and reacting at 60°C for 24h;
[0054] (2) Filter after 24 hours, wash the filter cake 6 times with water and ethanol successively, and place the washed precipitate at room temperature for 18 hours in vacuum to obtain 1.3145 g g-C 3 N 4 Base Na 2 B 12 h 12 ;
[0055] (3) 1.3124g g-C 3 N 4 Base Na 2 B 12 h 12 Disperse in 20ml of water; quickly add 0.0517g of sodium chloroaurate with a purity of 99%;
[0056] (4) After stirring and reacting at 40°C for 20 minutes under a 365nm ultraviolet lamp, filter to obtain a purple precipitate;
[0057] (5) After the precipitate was filtered, the precipitate was washed four times with water and ethanol in turn, and the washed precipitate was vacuum-dried at room temperature for 12 hours to obtain 1.3247 g g-C 3 N 4 Base Na 2 B 12 h 12 Nano gold catalyst;
[...
Embodiment 3
[0062] (1) 0.9956g g-C 3 N 4 Disperse in 50ml water, add 1.0765g Cs 2 B 6 h 6 , continuously stirring and reacting at 75°C for 72h;
[0063] (2) Filter after 72h, wash the filter cake 8 times with water and ethanol in turn, and place the washed precipitate at room temperature for 24h in vacuum to obtain 2.0043g g-C 3 N 4 Base Cs 2 B 6 h 6 , the material is characterized by Fourier transform infrared spectroscopy (FT-IR);
[0064] (3) 1.9475g g-C 3 N 4 Base Cs 2 B 6 h 6 Disperse in 20ml of water; quickly add 0.0682g of sodium chloropalladate with a purity of 99%;
[0065] (4) After stirring and reacting at 50°C for 30 minutes under a 365nm ultraviolet lamp, filter to obtain a black precipitate;
[0066] (5) After the precipitate was filtered, the precipitate was washed 8 times with water and ethanol in turn, and the washed precipitate was vacuum-dried at room temperature for 24 hours to obtain 2.2128g g-C 3 N 4 Base Cs 2 B 6 h 6 Nano palladium catalyst.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com