Forming process method for high-strength functional PCL/HA porous bone scaffold
A molding process and functional technology, applied in the field of bone tissue engineering and repair of large bone defects, can solve the problems of insufficient scaffold strength, scaffold shrinkage and deformation, and great influence on scaffold forming accuracy, so as to achieve controllable release and accelerated Effects on bone repair and reconstruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
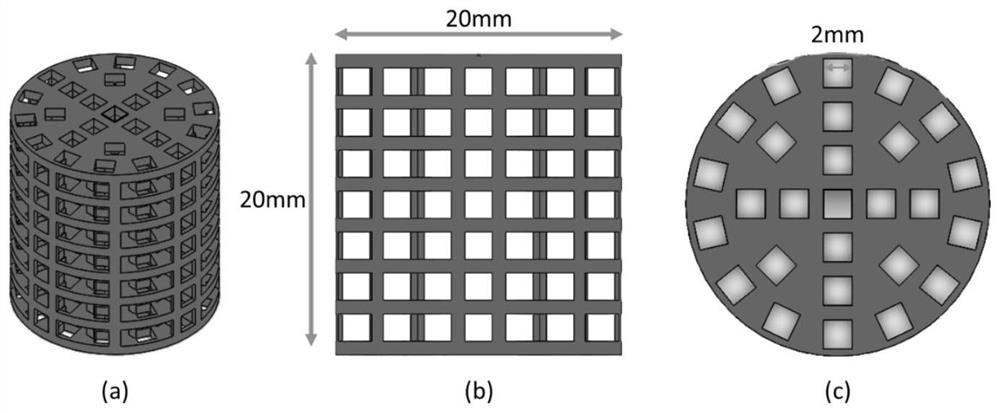
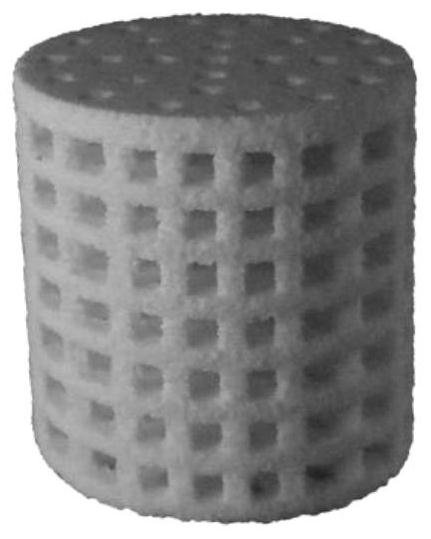
[0031]In this embodiment, the high-strength PCL / HA antibacterial porous bone scaffold is compositely printed by hydroxyapatite, polycaprolactone, polymer binder and gentamicin. In terms of mass percentage, the content of hydroxyapatite is 90%, the content of polycaprolactone is 10%, the polymer binder is 0.8% PVA solution, and the drug content is negligible. The specification of the stent is a cylinder with a diameter of 2 cm, and the interior of the stent is filled with through macroscopic pores, and the cross-section of the pore is a square of 2 mm×2 mm.
[0032] Specific steps are as follows:
[0033] 1. Weigh hydroxyapatite and polycaprolactone powder according to the given content (90% HA content, 10% PCL content), and mix them in a spiral mixer for 2 hours;
[0034] 2. Soak the mixture powder obtained in step 1 in 75% ethanol solution for 24 hours to sterilize, then wash it repeatedly with sterile PBS buffer solution for 5 times, dry it at room temperature for 4 hours a...
Embodiment 2
[0040] In this embodiment, the high-strength PCL / HA antibacterial porous bone scaffold is compositely printed by hydroxyapatite, polycaprolactone, polymer binder and gentamicin. In terms of mass percentage, the content of hydroxyapatite is 70%, the content of polycaprolactone is 30%, the polymer binder is 0.8% PVA solution, and the drug content is negligible. The specification of the stent is a cylinder with a diameter of 2 cm, and the interior of the stent is filled with through macroscopic pores, and the cross-section of the pore is a square of 2 mm×2 mm.
[0041] Specific steps are as follows:
[0042] 1. Weigh the hydroxyapatite and polycaprolactone powder according to the given content (HA content 70%, PCL content 30%), and mix them in a spiral mixer for 2 hours;
[0043] 2. Soak the mixture powder obtained in step 1 in 75% ethanol solution for 24 hours to sterilize, then wash it repeatedly with sterile PBS buffer solution for 5 times, dry it at room temperature for 4 ho...
Embodiment 3
[0050] In this embodiment, the high-strength PCL / HA antibacterial porous bone scaffold is compositely printed by hydroxyapatite, polycaprolactone, polymer binder and gentamicin. In terms of mass percentage, the content of hydroxyapatite is 95%, the content of polycaprolactone is 5%, the polymer binder is 0.8% PVA solution, and the drug content is negligible. The specification of the stent is a cylinder with a diameter of 2 cm, and the interior of the stent is filled with through macroscopic pores, and the cross-section of the pore is a square of 2 mm×2 mm.
[0051] Specific steps are as follows:
[0052] 1. Weigh hydroxyapatite and polycaprolactone powder according to the given content (HA content 95%, PCL content 5%), and place them in a spiral mixer for 2 hours;
[0053] 2. Soak the mixture powder obtained in step 1 in 75% ethanol solution for 24 hours to sterilize, then wash it repeatedly with sterile PBS buffer solution for 5 times, dry it at room temperature for 4 hours ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com