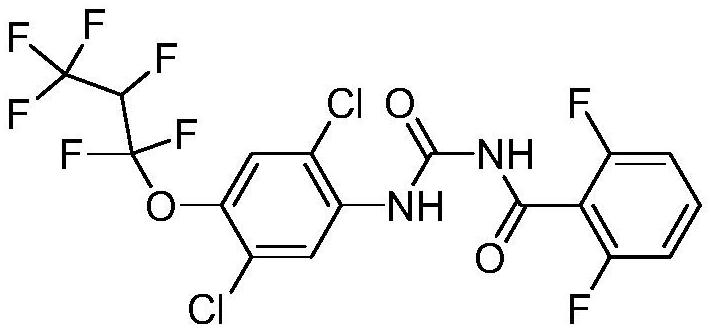
Synthesis method of benzamide pesticide lufenuron
A synthesis method and technology of benzamide are applied in chemical instruments and methods, preparation of ethers by addition of unsaturated compounds, preparation of urea derivatives, etc., which can solve the problems of difficulty in solvent recovery, affecting the quality of finished products, and generation of by-products, etc. Conducive to environmental protection, good product quality and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
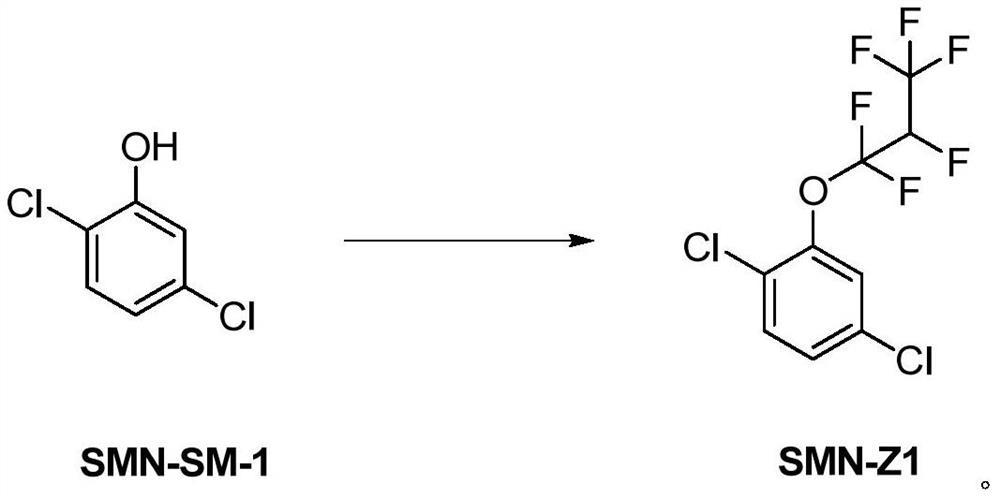
[0036] Preparation of SMN-Z1
[0037] In the 500L reactor, add 37.6kg of 2,5-dichlorophenol, 250kg of acetonitrile, 37kg of phenol, and 190kg of saturated sodium carbonate aqueous solution in sequence, check the tightness of the reactor, stir for 30 minutes, vacuumize -0.08MPa, and control the temperature of the cooling water at 20-25°C, ventilate hexafluoropropylene, the venting time is about 4.5-5.5 hours, and the ventilating speed is as long as the temperature does not exceed 25°C; the reaction kettle does not display positive pressure. After the aeration is over, keep warm for 1-1.5 hours, and the sampling gas phase control is qualified, and the reaction is ended. Post-processing: separate layers, the water layer is used as alkaline water, the oil phase is first concentrated at normal pressure to recover acetonitrile to 80°C, then concentrated acetonitrile to 115°C under reduced pressure, and the vacuum degree -0.08MPa to complete the distillation and cool down to obtain t...
Embodiment 2
[0039] Preparation of SMN-Z1
[0040] In the 500L reactor, add 37.6kg of 2,5-dichlorophenol, 250kg of acetonitrile, 50kg of phenol, and 190kg of saturated sodium carbonate aqueous solution in sequence, check the tightness of the reactor, stir for 30 minutes, vacuumize -0.08MPa, and control the temperature of the cooling water at 20-25°C, ventilate hexafluoropropylene, the venting time is about 4.5-5.5 hours, and the ventilating speed is as long as the temperature does not exceed 25°C; the reaction kettle does not display positive pressure. After the aeration is over, keep warm for 1-1.5 hours, and the sampling gas phase control is qualified, and the reaction is ended. Post-processing: separate layers, the water layer is used as alkaline water, the oil phase is first concentrated at normal pressure to recover acetonitrile to 80°C, then concentrated acetonitrile to 115°C under reduced pressure, and the vacuum degree -0.08MPa to complete the distillation and cool down to obtain t...
Embodiment 3
[0042] Preparation of SMN-Z1
[0043] In the 500L reactor, add 37.6kg of 2,5-dichlorophenol, 250kg of acetonitrile, 35.7kg of phenol, and 190kg of saturated sodium carbonate aqueous solution in sequence, check the tightness of the reactor, stir for 30 minutes, vacuumize -0.08MPa, and control the temperature with cooling water Hexafluoropropylene is ventilated at 20-25°C for about 4.5-5.5 hours, and the ventilating speed is as long as the temperature does not exceed 25°C; the reaction kettle does not show positive pressure. After the aeration is over, keep warm for 1-1.5 hours, and the sampling gas phase control is qualified, and the reaction is ended. Post-processing: separate layers, the water layer is used as alkaline water, the oil phase is first concentrated at normal pressure to recover acetonitrile to 80°C, then concentrated acetonitrile to 115°C under reduced pressure, and the vacuum degree -0.08MPa to complete the distillation and cool down to obtain the finished produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com