Single ion conductor polymer solid electrolyte membrane and preparation method and application thereof
A technology of solid electrolyte membrane and conductive polymer, which is applied in the field of electrochemical devices to achieve the effects of reducing concentration polarization, having good commercial prospects and improving cycle life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
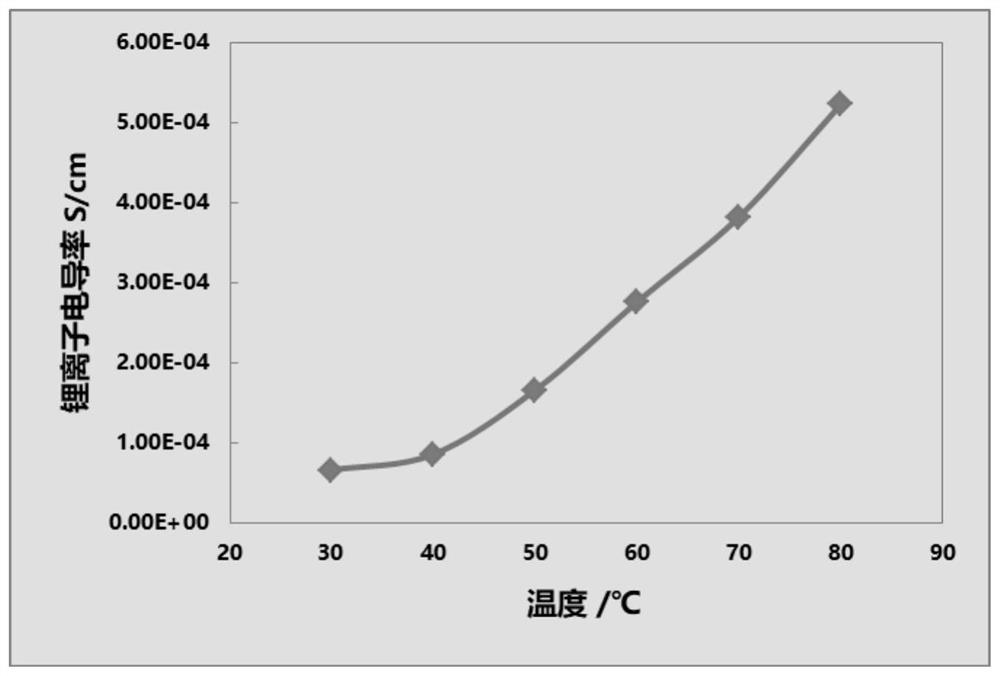
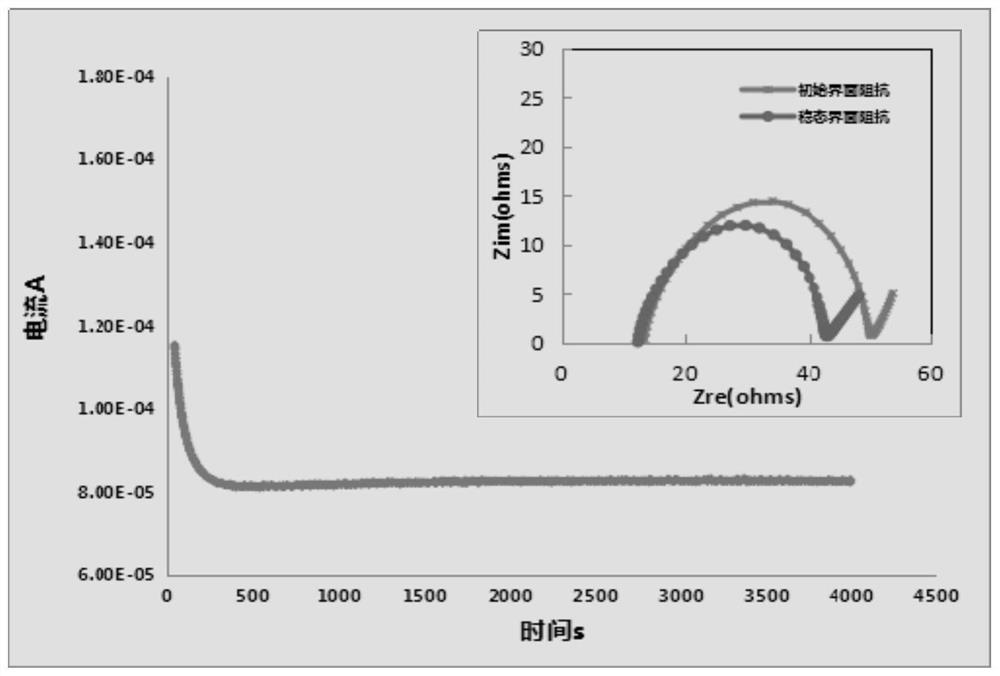
Image
Examples
Embodiment 1
[0059] (1) Synthesis of lithium-containing monomers with formula 2 structure
[0060] First, dissolve 0.35g of sodium dodecylsulfonate in 10mL of disodium hydrogen phosphate buffer solution (pH 4.5); then add 10mM polyetheramine and keep stirring until it is completely dissolved; finally add 0.5U / mL The laccase was used as a catalyst to initiate the polymerization reaction. Put the reaction container into a freezer at 5°C and let it stand for more than 24h. During the reaction, the suspension was tested by ultraviolet-visible light to monitor the progress of the reaction. After the reaction, an equal volume of acetone (10 mL) was added to the reaction solution to break the emulsion, and the resulting precipitate was collected by centrifugation and filtration, and washed with acetone and deionized water to remove oligomers and other impurities. The final product was dried in a drying oven at 60° C. to obtain terminal-modified sodium polyetherimide sulfonate.
[0061] Fully d...
Embodiment 2
[0076] The difference with embodiment 1 is:
[0077] (2) Preparation of Single Ion Conductor Polymer Solid Electrolyte Membrane
[0078] The lithium-containing monomer prepared by the above 27g, 15g crosslinking monomer trimethylolpropane triglycidyl ether, 15g lithium-ion-conducting polymer polyethylene oxide (PEO), 10g plasticizer tetraethylene glycol dimethyl ether , 7.5g inorganic particles Li 1.5 A l0.5 Ge 1.5 P 3 o 12 Dissolve and uniformly disperse in acetonitrile solvent in advance, add lithium salt LiTFSi, the addition ratio is 1:8 to the molar ratio of the EO group (that is, the lithium ion complex dissociation group) in the polymer and the monomer, and obtain the slurry .
Embodiment 3
[0080] The difference with embodiment 1 is:
[0081] (2) Preparation of Single Ion Conductor Polymer Solid Electrolyte Membrane
[0082]The lithium-containing monomer prepared by the above 10g, 20g crosslinking monomer trimethylolpropane triglycidyl ether, 2g lithium-ion-conducting polymer polyethylene oxide (PEO), 10g plasticizer tetraethylene glycol dimethyl ether , 10 g of inorganic particles Li 1.5 A l0.5 Ge 1.5 P 3 o 12 Dissolve separately in advance and evenly disperse in acetonitrile solvent, add lithium salt LiTFSi, the addition ratio is 1:16 to the molar ratio of the EO group in the polymer and the monomer (that is, the lithium ion complex dissociation group), to obtain a slurry .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com