Clindamycin hydrochloride injection and preparation method thereof
A technology for clindamycin hydrochloride and injection, which is used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. To avoid problems such as poor performance, to achieve the effects of prolonged storage time, good bacteriostatic effect and stable content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
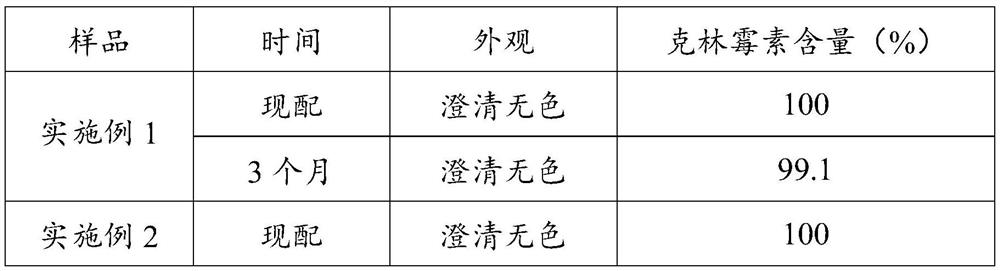
Examples
Embodiment 1
[0025] (1) Weigh 50 g of clindamycin hydrochloride, add water for injection to dissolve and dilute to 50 mL as stock solution, and set aside.
[0026] (2) Weigh 0.025 g of sodium edetate and dilute it with water for injection to obtain an aqueous solution of sodium edetate with a concentration of 0.1%.
[0027] (3) Accurately draw 25 mL of the stock solution, add 2.25 g of sodium chloride for injection, stir until dissolved, add 0.1% aqueous solution of sodium edetate to obtain a clindamycin hydrochloride mixed solution.
[0028] (4) Weigh 0.1 g of L-cysteine, dissolve it with water for injection, mix it with clindamycin hydrochloride mixed solution, and adjust the pH to 4.5 with pH 4.8 acetic acid-sodium acetate buffer solution.
[0029] (5) Heat the mixed solution to 115° C., and filter it with a 0.22 μm filter membrane under sterile conditions to obtain clindamycin hydrochloride injection.
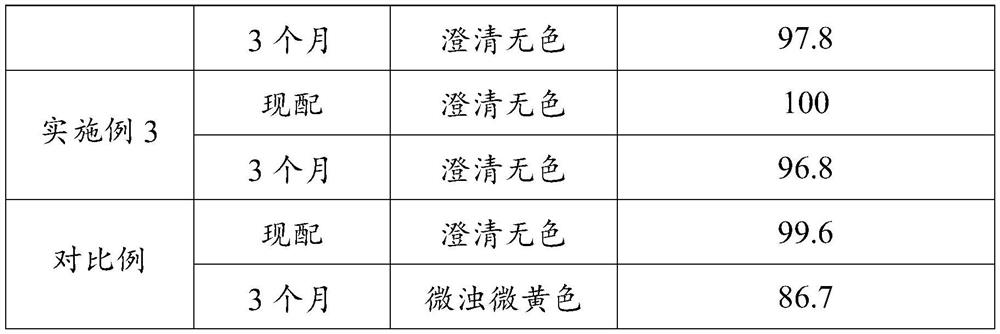
Embodiment 2
[0031] (1) Weigh 50 g of clindamycin hydrochloride, add water for injection to dissolve and dilute to 50 mL as stock solution, and set aside.
[0032] (2) Weigh 0.01 g of sodium edetate and dilute it with water for injection to obtain an aqueous solution of sodium edetate with a concentration of 0.05%.
[0033] (3) Precisely draw 25 mL of the stock solution, add 1.5 g of sodium chloride for injection, stir until dissolved, add 0.05% aqueous solution of sodium edetate to obtain a clindamycin hydrochloride mixed solution.
[0034] (4) Weigh 0.05 g of L-cysteine, dissolve it with water for injection, mix it with clindamycin hydrochloride mixed solution, and adjust the pH to 4 with pH 4.8 acetic acid-sodium acetate buffer solution.
[0035] (5) Heat the mixed solution to 115° C., and filter it with a 0.22 μm filter membrane under sterile conditions to obtain clindamycin hydrochloride injection.
Embodiment 3
[0037] Take 2 mL of the clindamycin hydrochloride injection prepared in Example 1, add 1 g of sucrose, place the mixed solution in liquid nitrogen for 10 min, and freeze-dry for 24 h using a lyophilizer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com