Antibacterial peptide, composition containing antibacterial peptide and application
A composition and antimicrobial peptide technology, applied in the direction of medical preparations containing active ingredients, medical preparations with non-active ingredients, antibacterial drugs, etc., can solve the problem of rapid activity reduction, difficulty in preparation, poor activity stability, etc. problem, to achieve the effect of low hemolysis rate and good antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
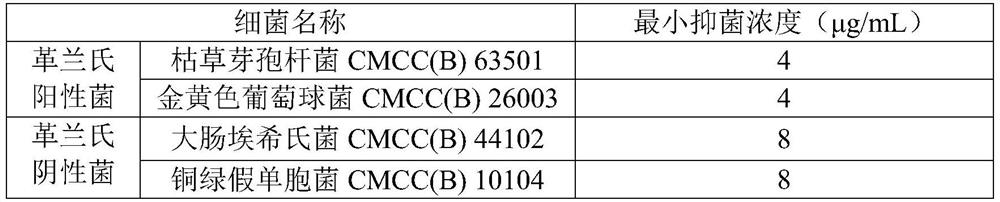
[0028] Bacteriostatic activity test
[0029] Add 100 μL of nutrient broth medium to each well of a 96-well plate, and then add 100 μL of an antimicrobial peptide aqueous solution (that is, only a mixed solution of antimicrobial peptide and water) with a concentration of 2048 μg / mL in the first row of the first column , mix the nutrient broth medium and antimicrobial peptide aqueous solution in the well evenly, draw 100 μL from the first row of the first column and add it to the second row of the first column, after mixing evenly, take 100 μL from the second row of the first column and add it to the next One row, repeat until the eighth row of the first column draws 100μL and discards; then dilute Staphylococcus aureus CMCC(B)26003 to 10 4 to 10 5 CFU / mL, inoculate 96-well plates with 100 μL bacterial solution per well, at this time, the final concentration of antimicrobial peptides in the first row of the first column is 1024 μg / mL, and the final concentration in the eighth r...
Embodiment 2
[0036] In vitro hemolysis rate test
[0037] The blood sample used in the detection of the hemolysis rate of animal erythrocytes by antimicrobial peptides is defibrinated sheep blood.
[0038] The detection method of the hemolytic activity rate of the antibacterial peptide is as follows: the erythrocytes are washed with PBS buffer solution (pH7.2, purchased from Beijing Soleibao Technology Co., Ltd.) and then centrifuged (1500r, 15min) to discard the supernatant, and the volume ratio is 92 The 0.9wt% sodium chloride aqueous solution of 8:8 mixed with the erythrocyte liquid after discarding the supernatant, was mixed with 8% erythrocyte suspension, got 8% erythrocyte suspension of 100 μ L in each well of 96 well plate, then to the first Add the antimicrobial peptide aqueous solution (the solution is prepared by antimicrobial peptide and purified water) to the first to third columns, so that the final concentrations of the antimicrobial peptides in the detection wells of the fir...
Embodiment 3
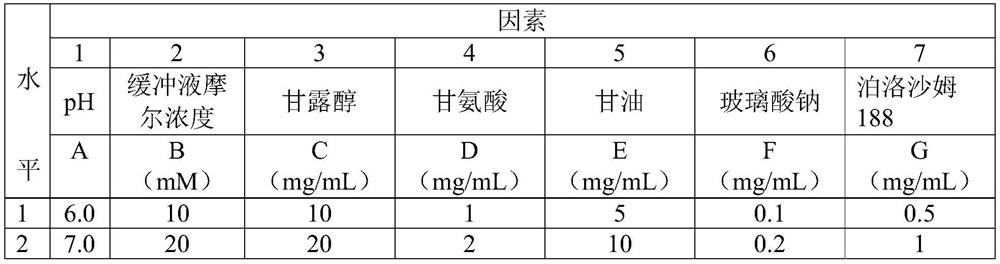
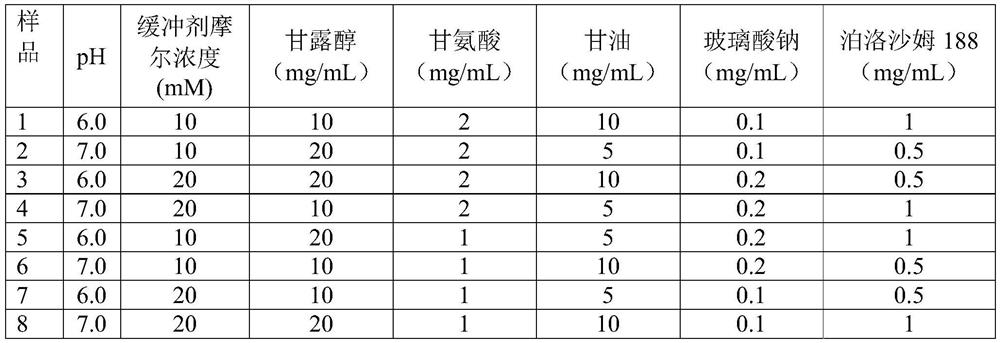
[0044] Sodium hyaluronate, glycerin, mannitol, glycine, poloxamer 188 and citrate buffer were selected, and an orthogonal experiment with 2 levels and 7 factors (see Table 3) was designed, and a total of 8 samples from sample 1 to sample 8 were obtained. The eye drops of the formula are specifically shown in Table 4, wherein the concentration of antimicrobial peptide in the eye drops is 1 mg / mL.
[0045] Table 3 Orthogonal design table with 2 levels and 7 factors
[0046]
[0047] Table 4 antimicrobial peptide eye drops obtained by orthogonal test
[0048]
[0049]The preparation process of antimicrobial peptide eye drops is as follows:
[0050] (1) Weigh sodium hyaluronate, glycine, glycerol and poloxamer 188 respectively according to Table 2.
[0051] (2) Add sodium hyaluronate, glycine, glycerin, and poloxamer 188 into sterile water for injection, and dissolve until there are no obvious white lumps, and filter the insoluble matter with a 0.22 μm filter membrane to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com