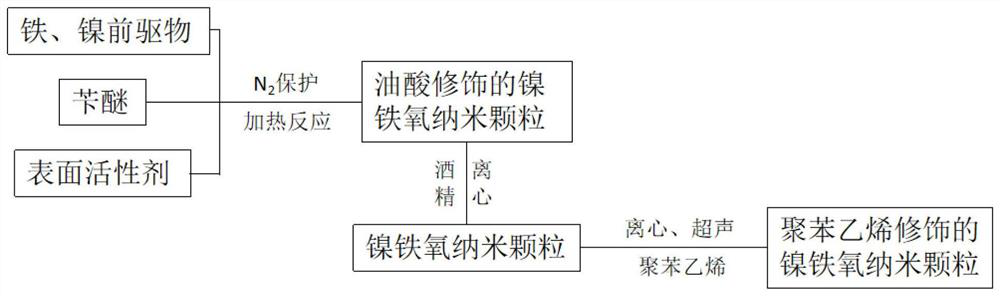
Controllable preparation and modification method of nickel ferrite nanoparticles
A nanoparticle, nickel ferrite technology, applied in the field of materials, can solve the problems of difficult storage, easy agglomeration and sedimentation of nanoparticles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1) Add 0.78g iron (III) acetylacetonate, 0.56g nickel (II) acetylacetonate, 0.9g sodium oleate, 6ml oleic acid, and 30mL benzyl ether solution into a 100mL three-necked reaction flask, stir to dissolve, and N 2 Under protection, the temperature was raised to 295° C. at a rate of 6° C. / min, and reacted for 1 hour to produce black nickel ferrite particle crystals with a mass of about 0.42 g.
[0037] 2) Take the above reaction product, use centrifugation, add an appropriate amount of n-hexane to wash, and then use centrifugation to repeat five times to obtain nickel ferrite nanoparticles coated with oleic acid.
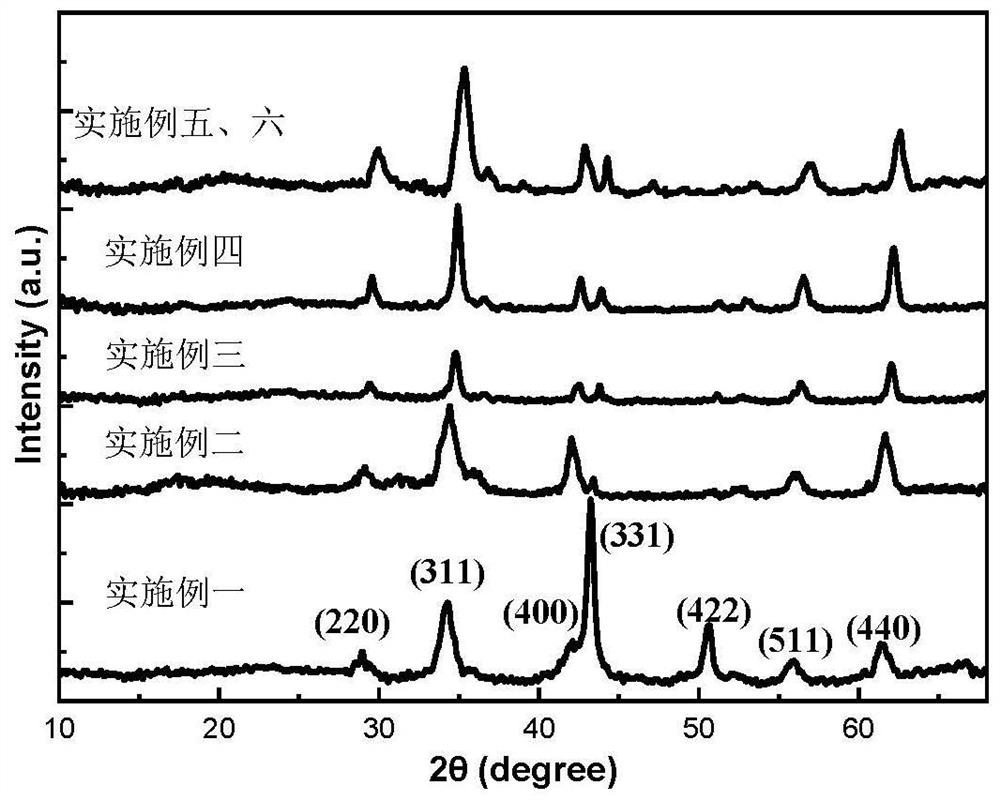
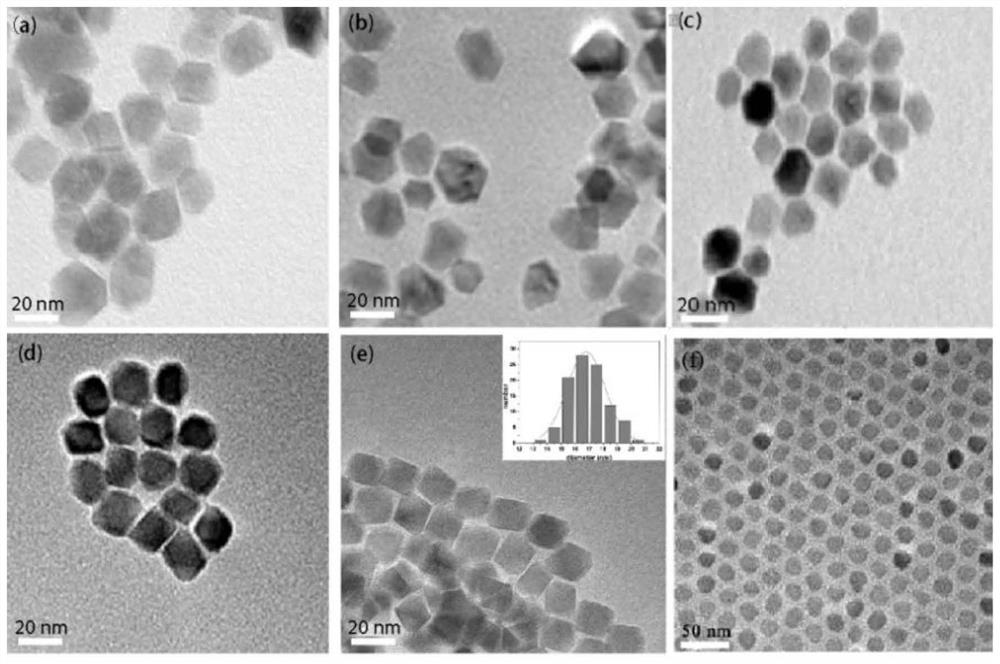
[0038] Such as figure 2 As shown, the X-ray diffraction pattern shows that the black solid prepared in the present embodiment is nickel ferrite nanoparticles; the transmission electron microscope image of preparing nanoparticles is as follows image 3 As shown in (a), it can be seen from the figure that the size distribution of the nanoparticles is relatively ...
Embodiment 2
[0042] 1) Add 1.162g iron (III) acetylacetonate, 0.283g nickel (II) acetylacetonate, 0.9g sodium oleate, 6ml oleic acid, and 30mL benzyl ether solution into a 100mL three-necked reaction flask, stir to dissolve, and N 2Under protection, the temperature was raised to 295° C. at a rate of 6° C. / min, and reacted for 1 hour to produce black nickel ferrite particle crystals with a mass of about 0.42 g.
[0043] 2) Take 1 mL of oleic acid-coated nickel ferrite nanoparticles (about 10 mg), use centrifugation, add an appropriate amount of n-hexane to wash, and use centrifugation to repeat five times.
[0044] Such as figure 2 As shown, the X-ray diffraction pattern shows that the black solid prepared in the present embodiment is nickel ferrite nanoparticles; the transmission electron microscope image of preparing nanoparticles is as follows image 3 As shown in (b), it can be seen from the figure that the size distribution of the nanoparticles is relatively uniform, and the diamete...
Embodiment 3
[0048] 1) Add 1.27g iron (III) acetylacetonate, 0.19g nickel (II) acetylacetonate, 0.9g sodium oleate, 6ml oleic acid, and 30mL benzyl ether solution into a 100mL three-necked reaction flask, stir to dissolve, and N 2 Under protection, the temperature was raised to 295° C. at a rate of 6° C. / min, and reacted for 1 hour to produce black nickel ferrite particle crystals with a mass of about 0.42 g.
[0049] 2) Take 1 mL of oleic acid-coated nickel ferrite nanoparticles (about 10 mg), use centrifugation, add an appropriate amount of n-hexane to wash, and use centrifugation to repeat five times.
[0050] Such as figure 2 As shown, the X-ray diffraction pattern shows that the black solid prepared in this embodiment is nickel ferrite nanoparticles; the transmission electron microscope image of the magnetic nickel ferrite nanoparticles body prepared is as follows image 3 As shown in (c), it can be seen from the figure that the size distribution of the nanoparticles is relatively ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com