A method for efficiently degrading environmental pollutants in a ferric iron/persulfate system by utilizing red phosphorus
A technology of environmental pollutants and persulfate, applied in the field of environmental pollutant treatment, can solve the problems of affecting PS activation, affecting degradation efficiency, poor cycle stability, etc., and achieve high PS utilization rate, high degradation efficiency, and wide application range of pH Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 A kind of novel PS activation system comprising red phosphorus
[0030] Take 2.0 g of red phosphorus and 18 mL of ultrapure water, mix well, and then place in a hydrothermal reactor at 200°C for hydrothermal reaction for 16 hours, filter, wash with water, and dry to obtain purified red phosphorus.
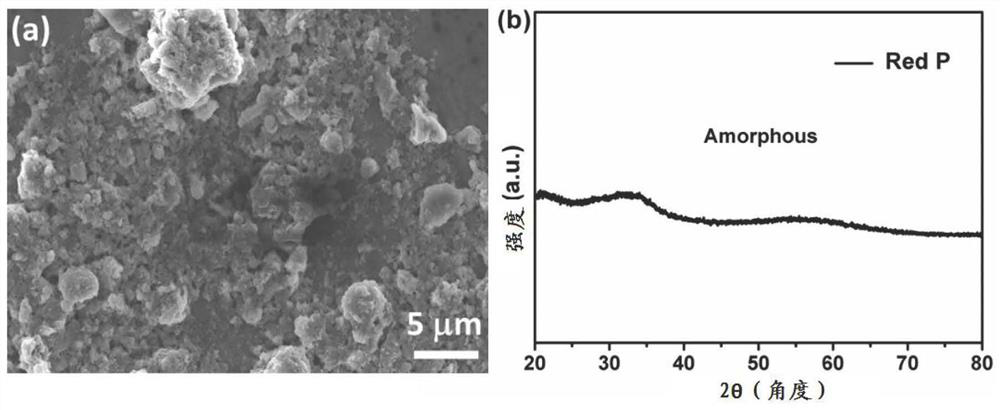
[0031] The purified red phosphorus was analyzed by scanning electron microscopy (SEM, Zeiss G-500) and X-ray diffraction (XRD, Rigaku SmartLab), and obtained from figure 1 It can be seen that the purified red phosphorus is irregular particles, and its lattice structure is Amorphous.
[0032]With purified red phosphorus (Red P) and potassium persulfate (PS), FeCl 3 Form a new PS activation system, namely RedP / PS / Fe 3+ system.
Embodiment 2
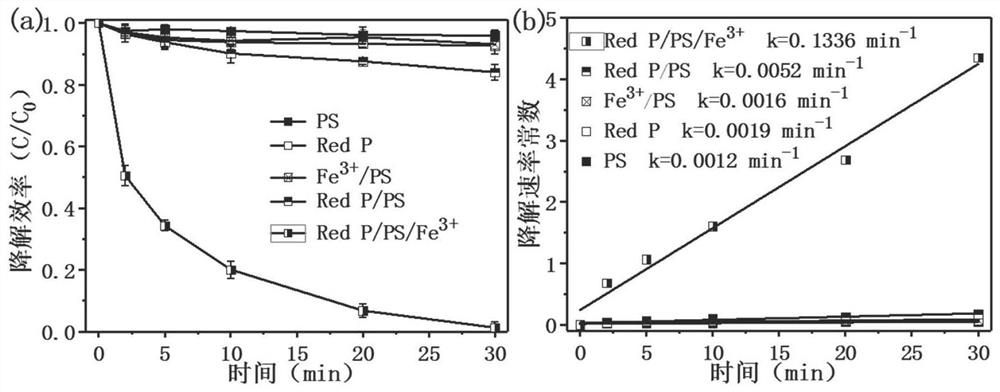
[0033] Example 2 Determination of Red P / PS / Fe 3+ Removal efficiency and kinetic constant of ibuprofen by the system
[0034] The measurement method is as follows:
[0035] (1) Prepare an ibuprofen solution with a concentration of 20 ppm.
[0036] (2) Take 60mL of ibuprofen solution in step (1) and transfer it into five 100mL beakers respectively, and the reaction systems of these five beakers are respectively set as: PS, Red P, Fe 3+ / PS, Red P / PS, Red P / PS / Fe 3+ . Among them, Red P, PS and Fe 3+ The dosing amounts of 0.2 g / L, 1 mM and 0.2 mM, respectively, were sampled on the set time gradient (0, 5, 10, 15, 20, 25, 30 min) for testing.
[0037] (3) Detect the ibuprofen concentration in the water sample of step (2) with high performance liquid chromatography (HPLC, Thermo Fisher Scientific), the detection wavelength is 223nm, and the mobile phase is phosphoric acid water (pH3.0) / methanol (20 / 80), the flow rate was 1 mL / min, and the peak time was 6.5 min. The calculati...
Embodiment 3
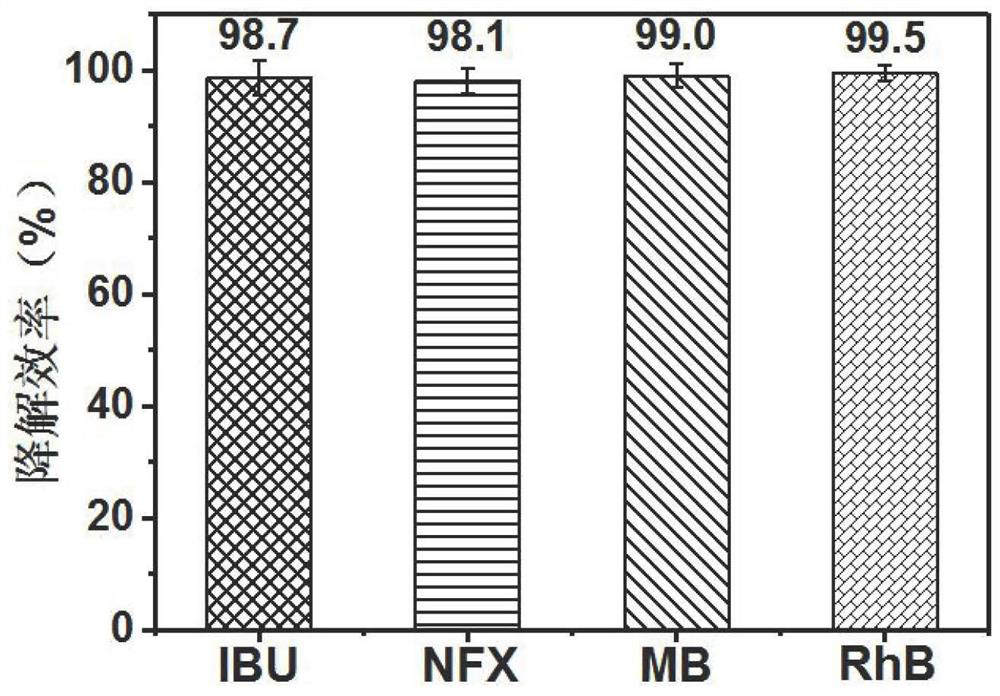
[0041] Example 3 Determination of Red P / PS / Fe 3+ The removal efficiency of the system for different pollutants
[0042] The measurement method is as follows:
[0043] (1) Prepare solutions of ibuprofen (IBU), norfloxacin (NFX), rhodamine B (RhB) and methyl blue (MB) with a concentration of 20 ppm.
[0044] (2) Take 60 mL of the four pollutant solutions in step (1) and transfer them to four 100 mL beakers, respectively, and then add 0.2 g / L red phosphorus, 1 mM PS and 0.2 mM Fe in sequence. 3+ , sample on the set time gradient (0, 5, 10, 15, 20, 25, 30 min) to be tested.
[0045] (3) Detect the ibuprofen concentration in the water sample of step (2) with high performance liquid chromatography (HPLC, Thermo Fisher Scientific), the detection wavelength is 223nm, and the mobile phase is phosphoric acid water (pH3.0) / methanol (20 / 80), the flow rate was 1 mL / min, and the peak time was 6.5 min. Use high performance liquid chromatography to detect the concentration of norfloxacin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com