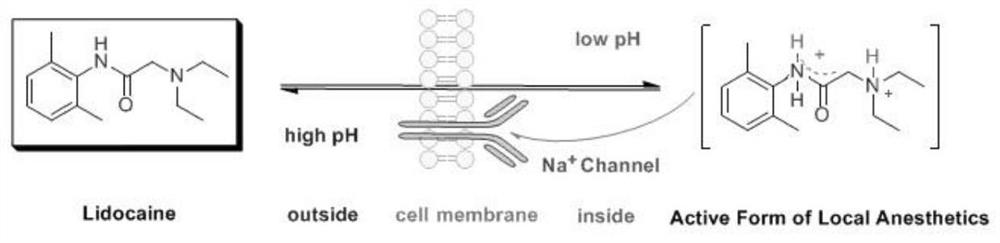
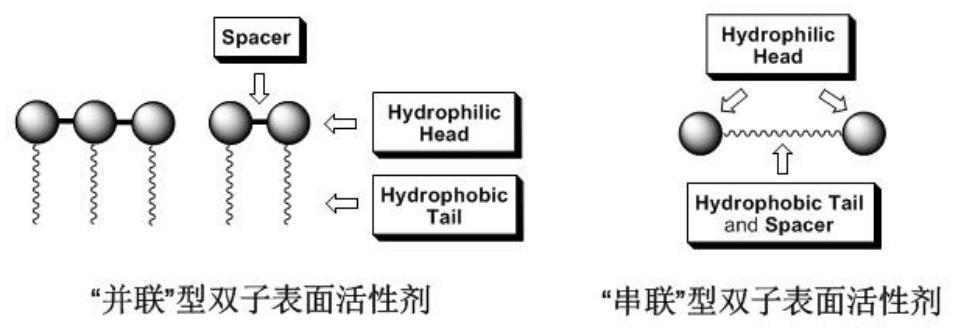
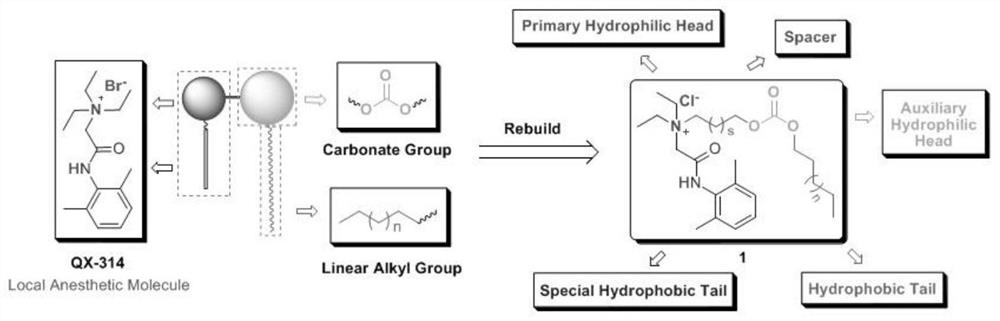
Arylamide compound based on benzene ring supramolecular interaction, self-assembly morphology, and use
A compound, the technology of dimethylanilinium, is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, organic chemistry, etc., and can solve problems such as unfavorable clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058]
[0059] In a 100mL round bottom flask, add 5mmol of sodium hydroxycarboxylate, 5mmol of pyridine, and 30mL of anhydrous acetonitrile, and stir for 0.5h under an ice bath. Slowly add 5mmol of the corresponding organic acid chloride solution in 25mL of anhydrous acetonitrile dropwise, and the drop is completed in 15min. After filtration, 10 mL of 1N ethanol solution of hydrogen chloride was added to the filtrate, and concentrated to dryness under reduced pressure. Dichloromethane-methanol silica gel column chromatography, concentration, vacuum drying, to obtain the corresponding carboxylic acid, as follows:
[0060]
Embodiment 2
[0062]
[0063] method one:
[0064] In a 100 mL round bottom flask, add 5 mmol of sodium hydroxycarboxylate and 30 mL of 1,2-dichloroethane. 2 mmol of trimeric phosgene was added under stirring at room temperature. Pyridine 5mmol was slowly added dropwise, and stirred at room temperature for 1h. Add 5mmol of the corresponding fatty alcohol or fatty amine dropwise, and stir at 50°C for 16h. Cool to room temperature and filter. Add 10 mL of 1N ethanol solution of hydrogen chloride to the filtrate, and concentrate to dryness. Dichloromethane-methanol silica gel column chromatography, concentration, vacuum drying, to obtain the corresponding carboxylic acid, as follows:
[0065]
[0066] Method Two:
[0067] In a 100 mL round bottom flask, add 5 mmol of sodium hydroxycarboxylate and 30 mL of 1,2-dichloroethane. Add 10 mL of 1,2-dichloroethane with 5 mmol of thionyl chloride dropwise under stirring in an ice bath, and drop it in 15 minutes. Remove the ice bath and sti...
Embodiment 3
[0070]
[0071] In a 50mL round bottom flask, add 10mmol of hydroxycarboxylic acid acetamide and 10mmol of fatty alcohol, add 10mL of 70% sulfuric acid with stirring at room temperature, stir and heat at 80°C for 4h. After cooling to room temperature, the residue was poured into a beaker filled with about 100 mL of ice-water mixture, and extracted with 50 mL x 4 dichloromethane. The organic phases were combined, washed with 20 mL x 2 water, dried over anhydrous sodium sulfate, and concentrated. Add 4mmol / L sodium hydroxide aqueous solution to the residue, and stir at reflux for 6h. The residue was acidified with 1N hydrochloric acid to pH=5.0, extracted with 50 mL x 4 dichloromethane, and concentrated. Dichloromethane-methanol silica gel column chromatography, concentrated, vacuum-dried to obtain the corresponding carboxylic acid as follows:
[0072]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com