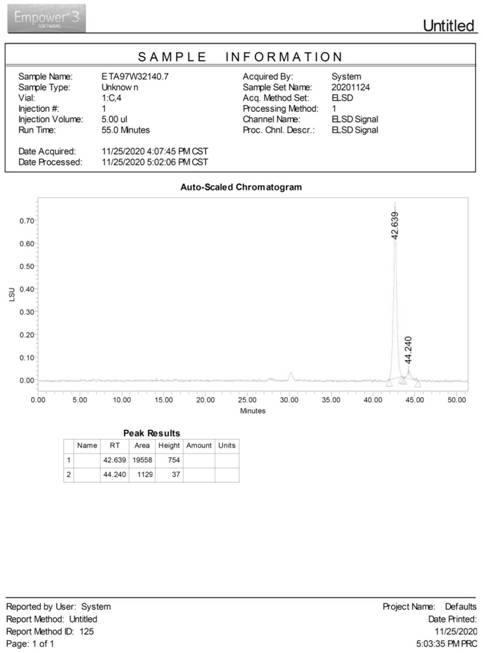
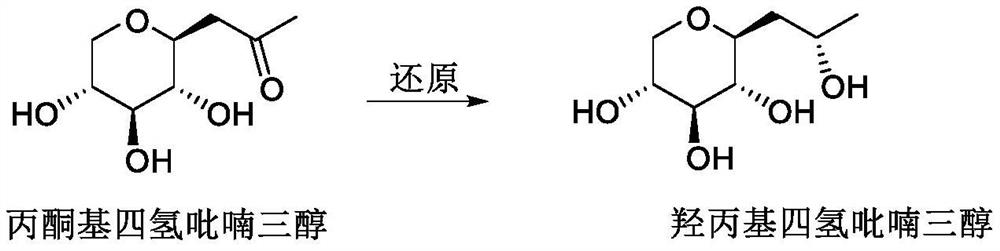
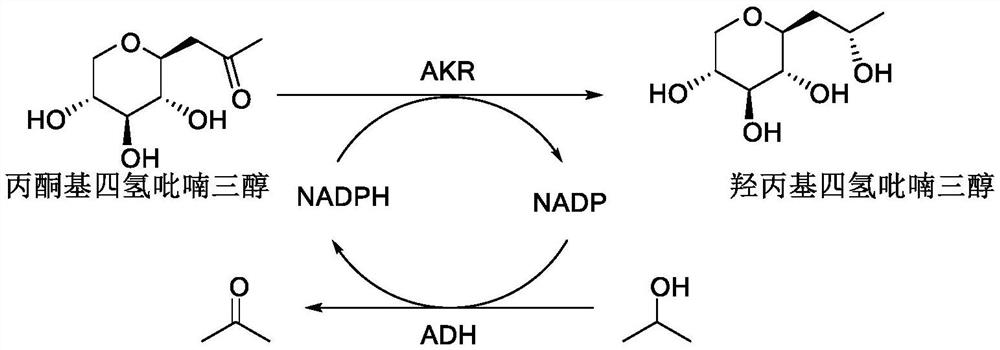
Synthetic method of hydroxypropyl tetrahydropyrantriol catalyzed by biological enzyme
A technology of hydroxypropyltetrahydropyrantriol and acetone-based tetrahydropyrantriol is applied in the synthesis field of cosmetic active substance hydroxypropyltetrahydropyrantriol, and can solve the problem of low stereoselectivity and environmental problems. Serious pollution, potential safety hazards and other problems, to achieve the effects of high purity, high enzyme conversion rate, and no metal residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1 (medium and reagent configuration)
[0015] (1) Luria-Bertani (LB) medium: Tryptone 10g L -1 , Yeast extract 5g·L -1 , NaCl 10 g·L -1 , Agarose 20g·L -1 (for solid medium), 5.0mol·L -1 Adjust the pH to 7.0 with NaOH (approximately 1‰) and sterilize at 121°C for 20min.
[0016] (2) 50mg·mL -1 Ampicillin solution (Amp): Dissolve 1 g of ampicillin in 20 mL of sterilized deionized water, filter and sterilize with a 0.22 μm filter membrane (Agela), dispense into sterile eppendorf tubes, 1 mL each, and store at -20°C for later use. Before use, add an amount of 1‰, that is, the final concentration is 50μg·mL -1 .
[0017] (3) 50mg·mL -1 Kanamycin Solution (Kan): Dissolve 1g Kanamycin in 20mL sterile deionized water, filter and sterilize with a 0.22μm filter membrane (Agela), dispense into sterile eppendorf tubes, 1mL each, -20°C Save for later. Before use, add an amount of 1‰, that is, the final concentration is 50μg·mL -1 .
[0018] (4) 200mg·mL -1 I...
Embodiment 2
[0030] Embodiment 2 (amplification of target gene)
[0031] The primers required for the PCR of aldehyde and ketone reductase AKR and alcohol dehydrogenase ADH were designed, and NdeI and XhoI restriction sites were respectively inserted at the carbon and nitrogen ends of the target gene by PCR.
[0032] Primer design
[0033]
[0034] Design primers and perform PCR operation. During PCR operation, a total reaction system of 50 μL is used. The system composition is as follows
[0035]
[0036] PCR conditions: ①Pre-denaturation at 98°C, 2min;
[0037] ② Denaturation at 98°C, 10s; annealing at 58°C, 5s; extension at 72°C, 5s / kb; cycle 35 times;
[0038] ③ Extend at 72°C for 5 minutes;
[0039] ④ 4°C, 1h (PCR can be terminated at any time at this stage).
Embodiment 3
[0040] Embodiment 3 (construction of pET-28a (+)-adh and pET-28a (+)-akr recombinant plasmid)
[0041] After the PCR product (ADH gene and AKR gene) was digested with double enzymes (NdeII and XhoI), it was ligated with the same double digested plasmid pET-28a(+) at 22°C for 2 hours; Transformed into E.coli DH5α competent, after sequencing and identification, the recombinant plasmids were then transferred into E.coli BL21(DE3), and the recombinant plasmids were named pET-28a(+)-adh and pET-28a(+)-akr , and then stored in a -80°C refrigerator.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com