Waste battery lithium resource recovery method based on solid electrolyte
A solid-state electrolyte, waste battery technology, used in battery recycling, waste collector recycling, secondary batteries, etc., can solve the problems of high impurities, high energy consumption at high temperature, slow kinetic rate, etc., and achieve high-purity lithium resource recovery. , the effect of improving lithium recovery efficiency and compensating for cost consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
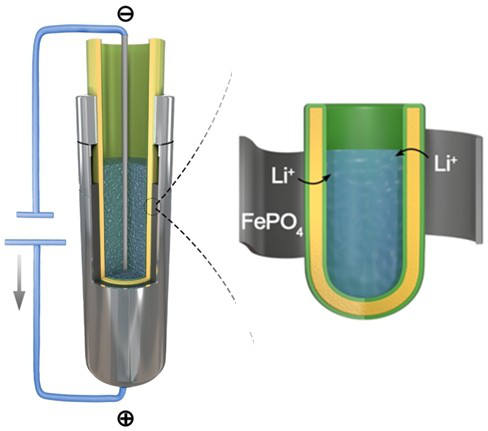
[0023] Such as figure 1 As shown, the method for recovering lithium resources from waste batteries based on solid electrolytes in this embodiment, the specific steps of the method are as follows:
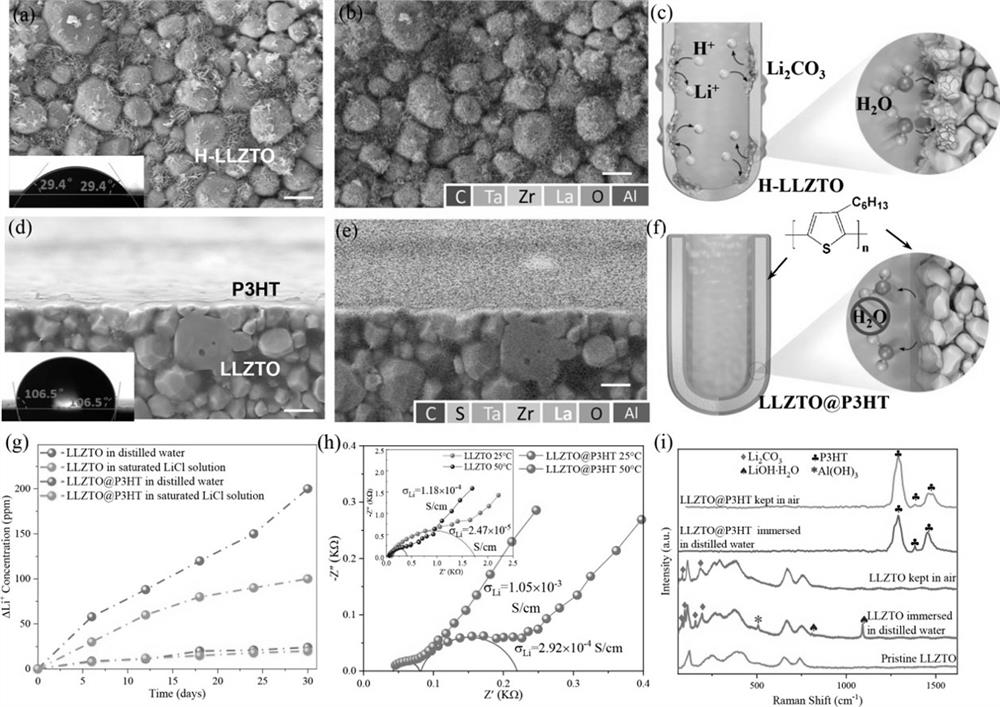
[0024] S1. Preparation of LLZTO waterproof ceramic tube: In a fume hood, dissolve a certain amount of P3HT powder in carbon disulfide solution, wherein the ratio of P3HT powder to carbon disulfide solution is 12mg / ml, and make a modified solution after sealing and stirring, and then LLZTO The ceramic tube is soaked in the modified solution for 5 minutes, and the soaked LLZTO ceramic tube is placed in a vacuum drying oven for drying. After drying, a waterproof coating will be formed on the surface of the LLZTO;
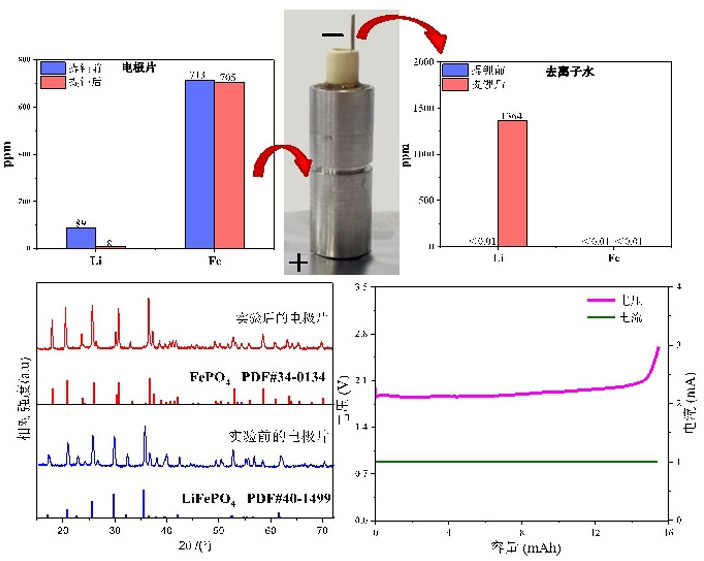
[0025] S2. In the glove box, wet the electrode sheet of the waste battery taken out through the lithium ion electrolyte for use. The lithium ion electrolyte is 1M lithium hexafluorophosphate dissolved in ethylene carbonate with a volume ratio of 1:1, mixed with diethyl carb...
Embodiment 2
[0033] In the method for recovering lithium resources from waste batteries based on solid electrolytes in this embodiment, the specific steps of the method are as follows:
[0034] S1. Preparation of LLZTO waterproof ceramic tube: In a fume hood, dissolve a certain amount of P3HT powder in chloroform solution, wherein the ratio of P3HT powder to chloroform solution is 22mg / ml, and make a modified solution after sealing and stirring, and then LLZTO The ceramic tube is soaked in the modified solution for 5 minutes, and the soaked LLZTO ceramic tube is placed in a vacuum drying oven for drying. After drying, a waterproof coating will be formed on the surface of the LLZTO;
[0035] S2. In the glove box, wet the electrode sheet of the waste battery taken out through the lithium ion electrolyte for use. The lithium ion electrolyte is 1M lithium hexafluorophosphate dissolved in ethylene carbonate with a volume ratio of 1:1, mixed with diethyl carbonate;
[0036] S3, in the glove box...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com