Organic light-emitting material as well as preparation method and application thereof
A light-emitting material and organic technology, which is applied in the preparation and application of light-emitting materials and organic compounds, can solve the problems of low molecular conjugation, and achieve the effect of simple purification method and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of TMAB crystals
[0047] At 60°C, add 2g of commercially available TMAB solid powder into the reactor, stir with 50ml of ethanol to make it dissolve as much as possible, and slowly add an appropriate amount of ethanol to the reactor under stirring to completely dissolve the TMAB solid, then let it stand for cooling. It slowly precipitated crystals, separated by suction filtration, and vacuum-dried to obtain TMAB crystals.
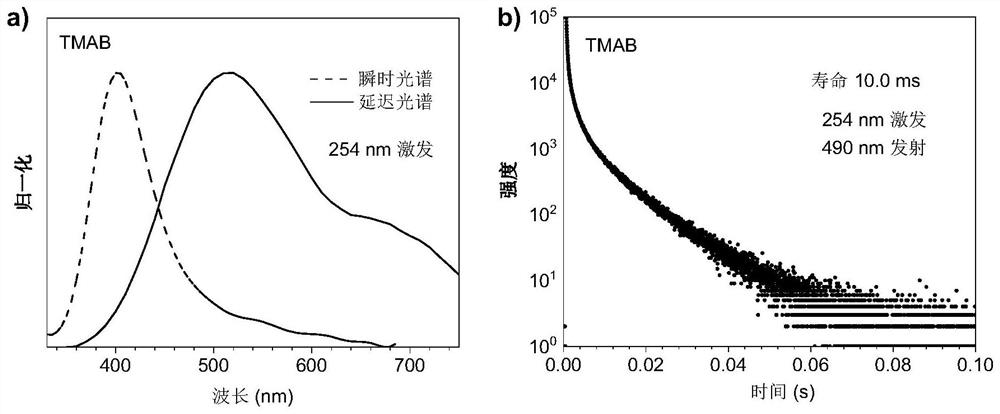
[0048] figure 2 It is the spectrum diagram of crystalline TMAB excited by (a) 254nm at room temperature, wherein the dotted line is the fluorescence spectrum, and the solid line is the phosphorescence spectrum (delay = 1ms); the lifetime diagram excited by (b) 254nm.
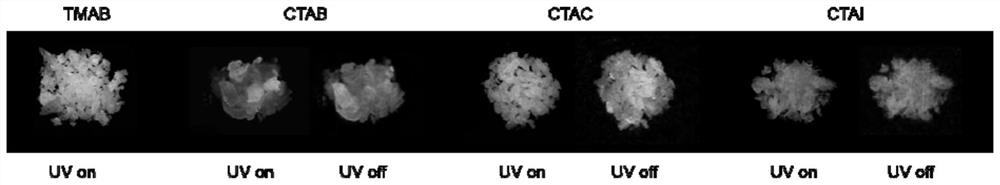
[0049] At room temperature, under the irradiation of 254nm ultraviolet rays, TMAB crystals have blue-white luminescence, from figure 2 It can be seen from the spectral test results at medium room temperature that the maximum fluorescence emission peak of TMAB is 400nm. ...
Embodiment 2
[0051] Preparation of CTAB crystals
[0052] At 60°C, add 2g of commercially available TMAB solid powder into the reactor, stir with 10ml of ethanol to make it dissolve as much as possible, and slowly add an appropriate amount of ethanol to the reactor under stirring to completely dissolve the TMAB solid, then let it stand for cooling, and wait for It slowly precipitated crystals, separated by suction filtration, and vacuum dried to obtain a purified CTAB crystal sample.
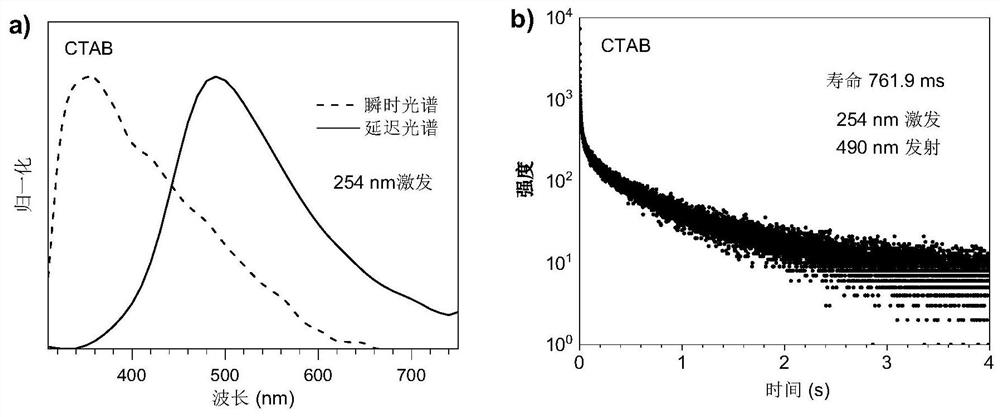
[0053] image 3 It is the spectrum diagram of crystalline CTAB excited by (a) 254nm at room temperature, wherein the dotted line is the fluorescence spectrum, and the solid line is the phosphorescence spectrum (delay = 1ms); the lifetime diagram excited by (b) 254nm.
[0054] At room temperature, under the irradiation of 254nm ultraviolet rays, CTAB crystals have blue luminescence, and after turning off the ultraviolet light, a green afterglow can be observed, from image 3 From the spectral test results a...
Embodiment 3
[0056] Preparation of CTAC crystals
[0057] At room temperature, add 2g of commercially available CTAC solid powder to a 25ml sample bottle, stir with 10ml of methanol to dissolve it completely, place the sample bottle in a 100ml jar filled with 20mL of anhydrous ether, and let the ether slowly volatilize into the sample bottle, after a large amount of crystals are precipitated. Separation by suction filtration and vacuum drying to obtain a purified CTAC crystal sample.
[0058] Figure 4 It is the spectrum diagram of crystalline CTAC excited by (a) 254nm at room temperature, where the dotted line is the fluorescence spectrum, and the solid line is the phosphorescence spectrum (delay = 0.1ms); the lifetime diagram excited by (b) 254nm.
[0059] At room temperature, under the irradiation of 254nm ultraviolet rays, CTAC crystals emit blue light, and after turning off the ultraviolet light, a green afterglow can be observed, from Figure 4 From the spectral test results at me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com