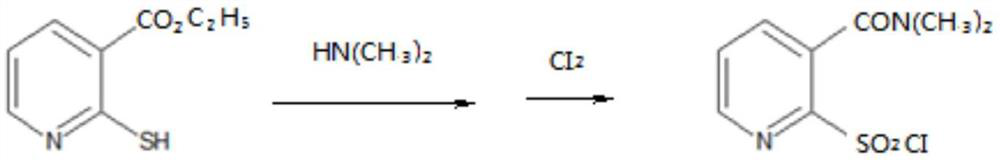
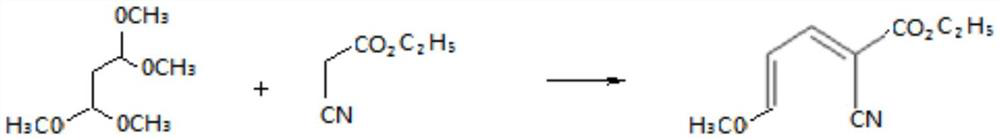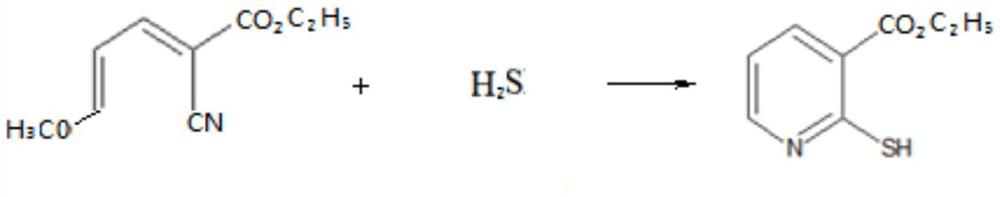Method for closed-loop synthesis of nicosulfuron by using hydrogen sulfide
A technology of nicosulfuron and hydrogen sulfide, applied in the direction of organic chemistry, etc., can solve the problems of large amount of waste water, complex production process, poor controllability of process safety, etc., achieve high comprehensive utilization rate of resources, simple process equipment, improved The effect of the production environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Step a: Preparation of cyanoalkene intermediate
[0051] Mix 16.4 grams (0.1mol) of tetramethoxypropane with 11.3 grams (0.1mol) of ethyl cyanoacetate, add 0.16 grams of zinc chloride as a catalyst, and then add 65 grams of methanol, heat to 65 ° C for 6 hours, and the reaction is complete , Atmospheric distillation first, distill out methanol for mechanical use, then distill out unreacted raw materials under reduced pressure, and then collect cyano-alkene. Its purity as determined by gas chromatography is 95.5%, and the yield is 95.2%;
[0052] Step b: Preparation of thiols
[0053] Take 18.9 grams of the cyanoalkene intermediate obtained in step a (purity 95.5%, 0.1 mol) and add it to the reaction flask, add 150 milliliters of dichloroethane, start stirring and cool down to 10 ° C, slowly start to feed hydrogen sulfide gas, The reaction temperature is controlled at 10-15°C. During the reaction process, samples are continuously taken for analysis until the remaining ...
Embodiment 2
[0061] Step a: Preparation of cyanoalkene intermediate
[0062] Mix 16.4 grams (0.1mol) of tetramethoxypropane with 12.4 grams (0.11mol) of ethyl cyanoacetate, add 0.3 grams of catalyst zinc chloride, then add 65 grams of methanol, heat to 65 ° C for 6 hours, and the reaction is complete , Atmospheric distillation first, distill out methanol for mechanical use, then distill out unreacted raw materials under reduced pressure, and then collect cyano-alkenes. Its purity as determined by gas chromatography is 96.2%, and the yield is 95.9%;
[0063] Step b: Preparation of thiols
[0064] Take 18.9 grams of the cyanoalkene intermediate obtained in step a (purity 95.5%, 0.1 mol) and add it to the reaction flask, add 150 milliliters of dichloroethane, start stirring and cool down to 10 ° C, slowly start to feed hydrogen sulfide gas, The reaction temperature is controlled at 10-15°C. During the reaction process, samples are continuously taken for analysis until the remaining cyanoalk...
Embodiment 3
[0072] Step a: Preparation of cyanoalkene intermediate
[0073] Mix 16.4 grams (0.1mol) of tetramethoxypropane with 11.3 grams (0.1mol) of ethyl cyanoacetate, add 0.16 grams of catalyst zinc chloride, then add 70 grams of ethanol, heat to 80 ° C for 6 hours, and the reaction is complete , Atmospheric distillation first, distill out methanol for mechanical use, then distill out unreacted raw materials under reduced pressure, and then collect cyano-alkenes. Its purity as determined by gas chromatography is 95.9%, and the yield is 95.6%;
[0074] Step b: Preparation of thiols
[0075] Take 18.9 grams of the cyanoalkene intermediate obtained in step a (purity 95.5%, 0.1 mol) and add it to the reaction flask, add 150 milliliters of dichloroethane, start stirring and cool down to 10 ° C, slowly start to feed hydrogen sulfide gas, Control the reaction temperature at 70-75°C. During the reaction process, keep sampling and testing until the remaining cyanoalkene is less than 2%, stop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com