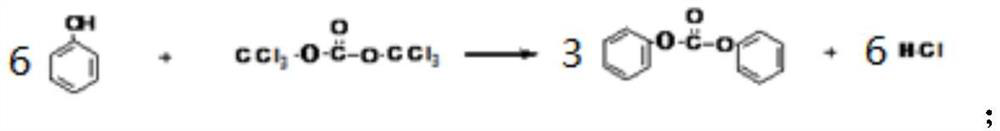
Method for circularly producing nicosulfuron active compound by using byproduct phenol
A technology of nicosulfuron original drug and nicosulfuron, which is applied in the preparation of phosgene or haloformate, organic chemistry, etc., and can solve the problems of high toxicity, low production efficiency, and large amount of waste water.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Step a: Preparation of sulfonamido-phenyl formate intermediate
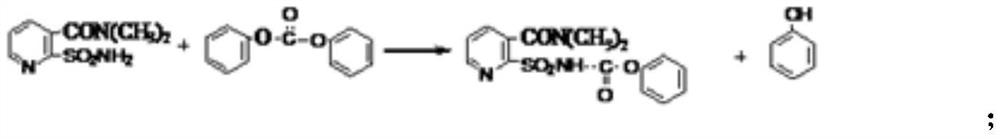
[0050] Add 23 grams (0.1mol) of N,N-dimethyl-2-aminosulfonyl-3-pyridinecarboxamide, then add 24 grams (0.11mol) of diphenyl carbonate and 5.6 grams (0.1mol) of potassium hydroxide, Add 150ml of acetone and 50ml of water, stir and heat to reflux for reaction, check the end point after 5 hours until the reaction is complete, distill out acetone and recover it, add 200g of tap water, control the temperature of the reaction solution at 30-35°C, add 30% hydrochloric acid dropwise Until the pH of the reaction solution reaches 1, after filtration, the mother liquor and filter cake containing by-product phenol are obtained, the filter cake is washed, and dried to a constant weight to obtain 35 grams of phenyl ester intermediate, which has a purity of 92.3% and a yield of 92.6%;
[0051] Step b: Preparation of Nicosulfuron
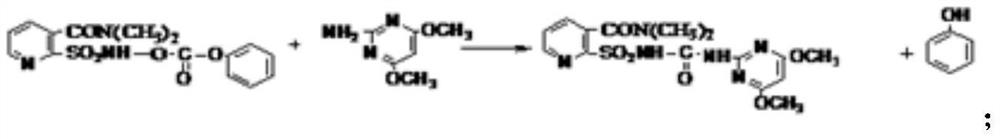
[0052] Take 18.9 grams (0.05mol) of the sulfonamido-phenylformate intermediate prepared in...
Embodiment 2
[0056] Step a: Preparation of sulfonamide-phenyl formate intermediate Add 23 grams (0.1mol) of N,N-dimethyl-2-aminosulfonyl-3-pyridinecarboxamide, then add 24 grams (0.11mol) of dicarbonate Phenyl ester and 5.6 gram (0.1mol) potassium hydroxide, add 150 milliliters of acetone and 50 milliliters of water, stir and heat reflux reaction, detect end point after 5 hours, until after reaction is finished, distill out acetone recovery and apply mechanically, add 200 grams of tap water, control The temperature of the reaction solution is 20-30°C, and 30% hydrochloric acid is added dropwise until the pH of the reaction solution reaches 1. After filtration, the mother liquor and filter cake containing by-product phenol are obtained. The filter cake is washed and dried to constant weight to obtain 35.2 grams of phenyl ester intermediate. Its purity is 92.5%, and its yield is 94.6%;
[0057] Step b: Preparation of Nicosulfuron
[0058] Take 18.9 grams (0.05mol) of the sulfonamido-phenylf...
Embodiment 3
[0062] Step a: Preparation of sulfonamido-phenyl formate intermediate
[0063] Add 23 grams (0.1mol) of N,N-dimethyl-2-aminosulfonyl-3-pyridinecarboxamide, then add 24 grams (0.11mol) of diphenyl carbonate and 5.6 grams (0.1mol) of potassium hydroxide, Add 150ml of acetone and 50ml of water, stir and heat to reflux reaction, check the end point after 5 hours, until the reaction is completed, distill out the acetone for recovery, add 200g of tap water, control the temperature of the reaction solution at 28-32°C, add 30% hydrochloric acid dropwise When the pH of the reaction liquid reached 1, the mother liquor and filter cake containing by-product phenol were obtained after filtration. The filter cake was washed and dried to constant weight to obtain 35.3 g of phenyl ester intermediate with a purity of 92.3% and a yield of 95.2%.
[0064] Step b: Preparation of Nicosulfuron
[0065] Take 18.9 grams (0.05mol) of the sulfonamido-phenylformate intermediate prepared in step a and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com