4-thiodeoxythymidine derivatives and their pharmaceutical application against hepatitis B virus
A technology of thiodeoxythymidine and derivatives, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problem of drug resistance, drug resistance needs to be improved, and hepatitis B virus cannot be completely eliminated. problem, to achieve the effect of cheap raw materials, easy large-scale production, and clever design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
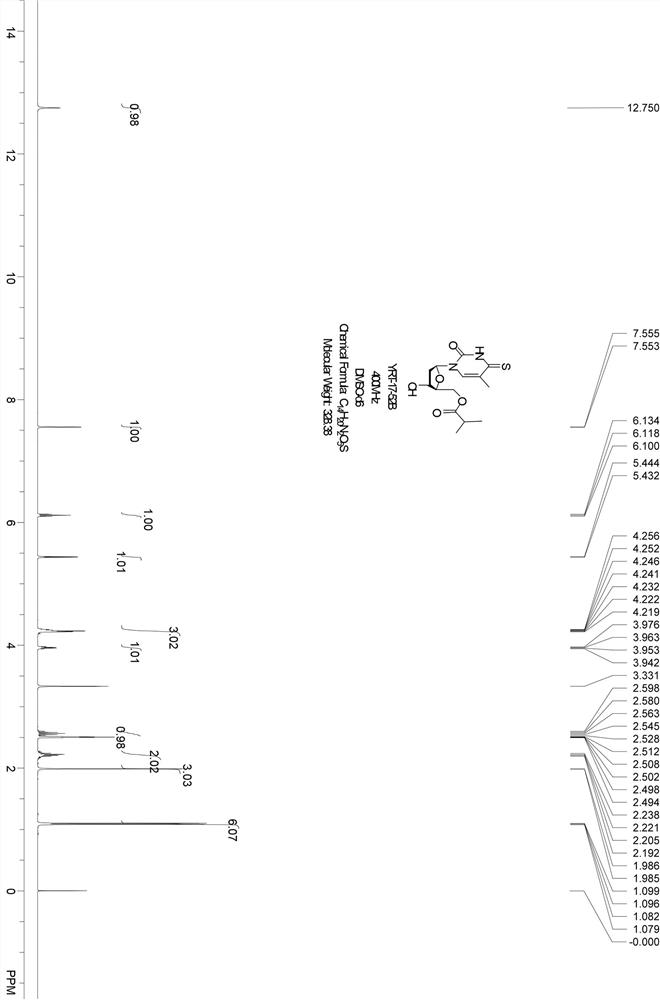
Embodiment 1
[0038] The synthetic route is as follows:
[0039] ;
[0040] Thymine (26 g, 205.7 mmol) in hexamethyldisilazane (320 mL, 1.48 mol) and trimethylchlorosilane (42 mL, 321 mmol), N 2 Under protection, stir at 130°C for 3h. After cooling to room temperature, the reaction solution was concentrated, and the oil pump was drained. Dichloromethane (500 mL, anhydrous) was added to the crude product, and then compound A (CAS: 141846-57-3) (80 g, 205.7 mmol) and trifluoro Trimethylsilyl methanesulfonate (2.25 mL, 12.3 mmol), stirred at room temperature for 1 h. After the reaction was completed, slowly add saturated sodium bicarbonate solution to the reaction solution under stirring, extract with dichloromethane, wash the organic phase with saturated brine, separate the organic phase, dry over anhydrous sodium sulfate, filter to remove anhydrous sodium sulfate, and the filtrate Concentration gave a crude product, which was separated by column chromatography (petroleum ether: ethyl ac...
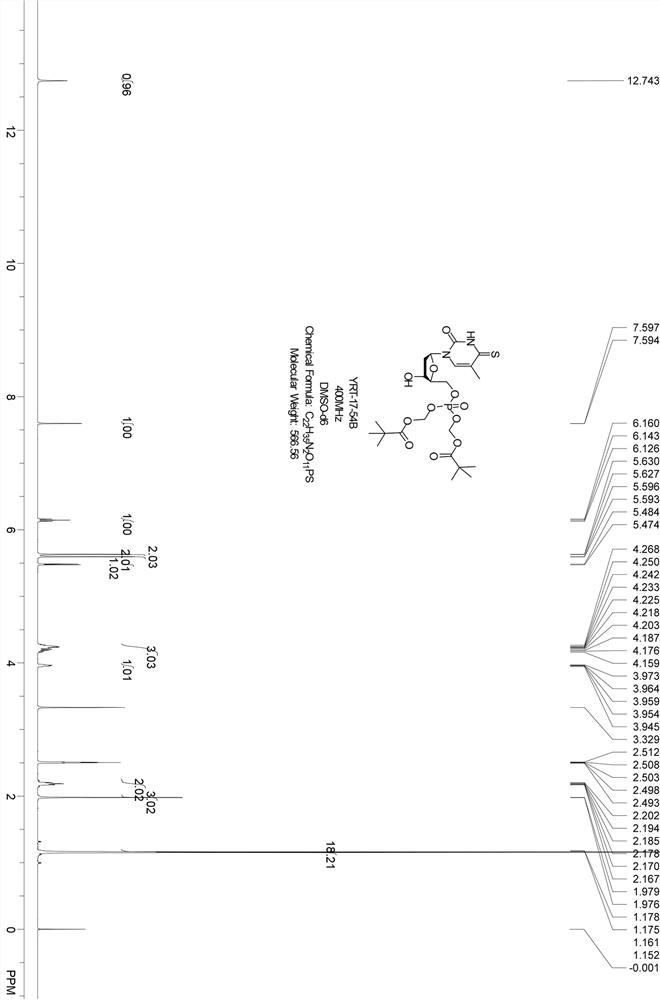
Embodiment 2
[0054] The synthetic route is as follows:
[0055] ;
[0056] Intermediate compound 1 (2 g, 3.8 mmol) and compound I (3.4 g, 7.5 mmol, CAS: 1354823-36-1) were dissolved in MeCN (100 mL), magnesium chloride (359 mg, 3.8 mmol) was added, and at 50 °C Stir for 10 min, then add DIEA (1.22 g, 9.4 mmol), and stir overnight at 50°C. After the reaction, cool to room temperature, add dichloromethane (200 mL) to dilute, wash the reaction solution with citric acid (1N), wash the organic phase with saturated ammonium chloride in turn, then wash with saturated sodium bicarbonate, and then with saturated brine, and wash the organic phase with It was dried over anhydrous sodium sulfate, filtered to remove anhydrous sodium sulfate, the filtrate was concentrated, and the crude product was separated by column chromatography (petroleum ether: ethyl acetate = 10 / 1-1 / 1) to obtain compound J (2.3 g, yellow solid).
[0057] 1 H NMR (400 MHz, DMSO- d 6 ) δ 12.76 (s, 1H), 7.42 (d,5H), 7.36 (dd, 4...
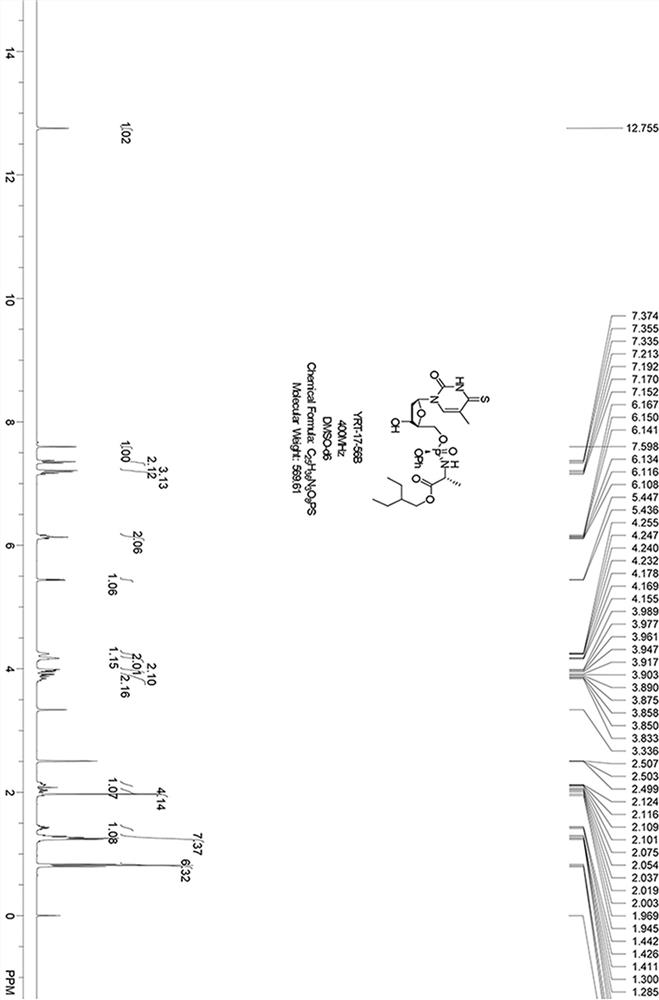
Embodiment 3
[0061] The synthetic route is as follows:
[0062] ;
[0063] Chloromethyl pivalate (48.2 g, 320 mmol), trimethyl phosphate (11.2 g, 80 mmol) and sodium iodide (36 g, 240 mmol) were mixed in acetonitrile (100 mL, anhydrous), and added to the reaction solution Add a few grains of 4A molecular sieve, N 2 90 under protection o C was refluxed overnight, after the reaction was completed, the reaction solution was cooled to room temperature, filtered with diatomaceous earth, the filter residue was washed with ethyl acetate, the filtrate was concentrated, and the crude product was separated by column chromatography (petroleum ether: ethyl acetate = 10 / 1-5 / 1) to obtain Product L (28.3 g, colorless liquid).
[0064] Compound L (6 g, 13.6 mmol) and lithium bromide (1.2 g, 13.6 mmol) were dissolved in acetonitrile (50 mL, dry), 90 o C was refluxed and stirred overnight. After the reaction was completed, it was cooled to room temperature, filtered, and the filter cake was washed wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com