Nanometer antibody aiming at green fluorescent protein (GFP) and application thereof
A technology of green fluorescent protein and nano-antibody, which is applied in the field of genetic engineering antibody and single-domain heavy chain antibody, can solve the problems of undiscovered patent publications, etc., and achieve high binding specificity and good application effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
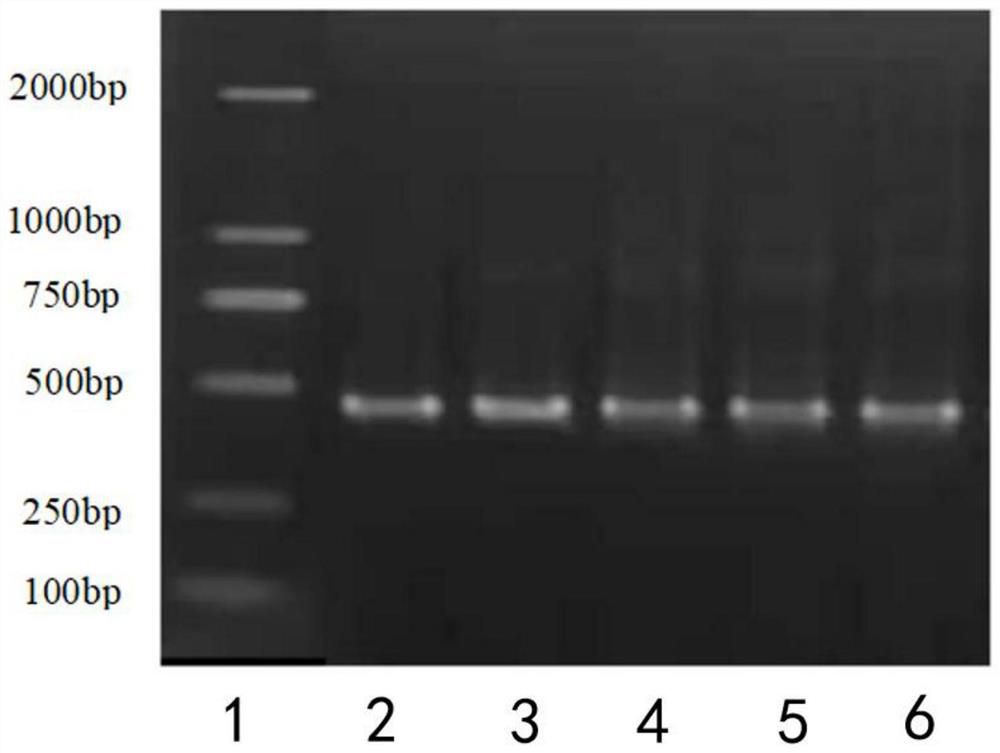
[0043] Example 1: Construction of a Nanobody library for GFP:
[0044] (1) The concentration of GFP is 500 micrograms per milliliter, and 1 mg of GFP is mixed with Freund's adjuvant in equal volume for each immunization, and a Xinjiang Bactrian camel is immunized once a week, a total of 4 times, except for the first use Complete Freund's adjuvant, and Freund's incomplete adjuvant for the remaining several times, stimulate B cells to express antigen-specific nanobodies during the immunization process.
[0045] (2) After the 4 times of immunization, extract 100 ml of camel peripheral blood lymphocytes and extract total RNA, referring to the RNA extraction kit provided by QIAGEN.
[0046](3) According to the instructions of Super-Script III FIRST STRANDSUPERMIX kit, the extracted RNA was reverse-transcribed into cDNA and the VHH chain was amplified by nested PCR. The first round of PCR:
[0047] Upstream primer: GTCCTGGCTGCTCTTCTACAAGGC
[0048] Downstream primer: GGTACGTGCTGTT...
Embodiment 2
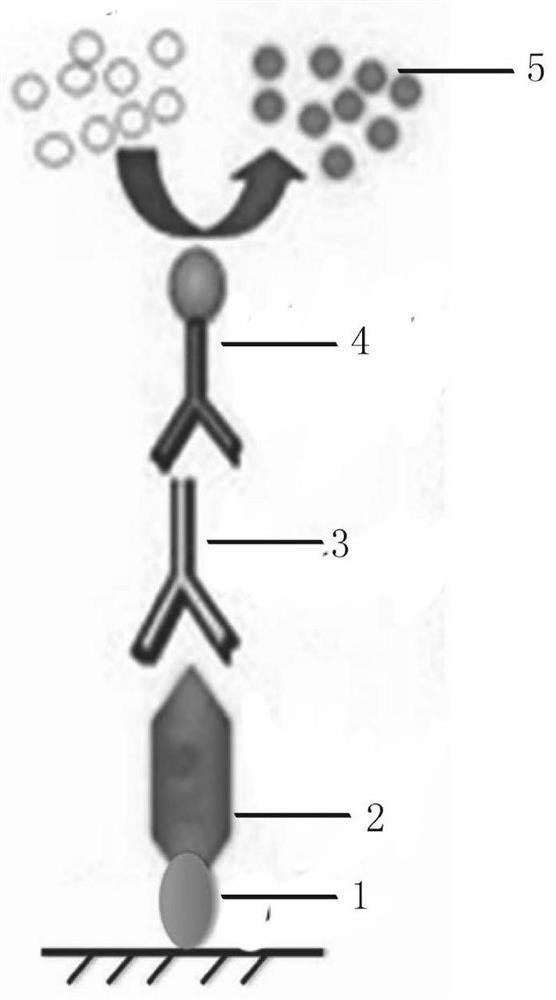
[0057] Embodiment 2: Nanobody screening process against GFP:
[0058] (1) Dissolve in 100mM pH 8.2NaHCO 3 200 micrograms of GFP in the medium were coupled to a microtiter plate, placed overnight at 4°C, and a negative control was set up at the same time.
[0059] (2) On the second day, 100 microliters of 0.1% casein were added to the two wells respectively, and blocked for 2 hours at room temperature.
[0060] (3) After 2 hours, add 100 μl phage (8*10 11 tfu immunized camel nanobody phage display gene library) and acted for 1 hour at room temperature.
[0061] (4) Wash 5 times with PBST (PBS containing 0.05% Tween 20) to wash away unbound phages.
[0062] (5) Use triethylamine (100mM) to dissociate the phage that specifically binds to GFP, and infect Escherichia coli TG1 that is in logarithmic growth, produce and purify the phage for the next round of screening, the same screening process Repeat for 3-4 rounds. In the process of continuous screening, positive clones will ...
Embodiment 3
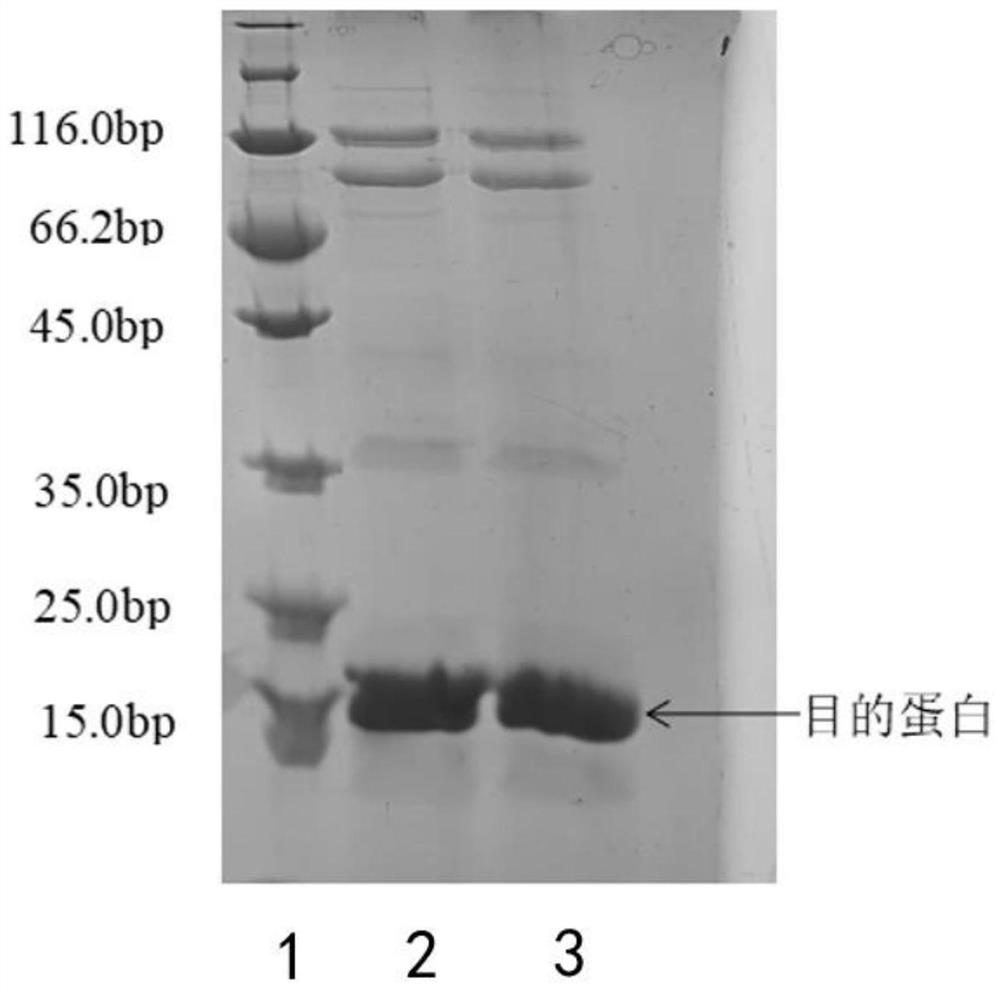
[0063] Embodiment 3: use the enzyme-linked immunosorbent method (ELISA) of phage to screen specificity single positive clone:
[0064] (1) From the cell culture dish containing phage after the above 3-4 rounds of selection, pick 96 single colonies and inoculate them in TB medium containing 100 micrograms per milliliter of ampicillin (1 liter of TB medium contains 2.3 grams of phosphoric acid Potassium dihydrogen, 12.52 grams of dipotassium hydrogen phosphate, 12 grams of peptone, 24 grams of yeast extract, 4 milliliters of glycerol), after growing to the logarithmic phase, add IPTG with a final concentration of 1 millimolar, and cultivate overnight at 28 ° C.
[0065] (2) Obtain the crudely extracted antibody by infiltration method, transfer the antibody to an antigen-coated ELISA plate, and place it at room temperature for 1 hour.
[0066] (3) Unbound antibodies were washed away with PBST, a mouse anti-HAtag antibody (anti-mouse anti-HA antibody, purchased from Beijing Kangwe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com