An antimicrobial peptide hehampii-2(63-86) and its application
An antibacterial peptide and nucleic acid technology, applied in the application, antibacterial, antifungal and other directions, can solve the problems of natural antibacterial peptide toxicity, eukaryotic cell hemolysis, narrow antibacterial peptide antibacterial spectrum, etc. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Determination of the sequence of the striae hippocampal antimicrobial peptide hepcidin (HeHamp II-2)
[0021] Based on the striae hippocampus genome and transcriptome database, the invention obtains the cDNA sequence of HeHamp II-2 by screening, and clones the antibacterial peptide sequence from striae hippocampus tissue. The specific steps are:
[0022] Total RNA was extracted from adult male hippocampus liver tissue using TRIzol reagent (Invitrogen, Waltham, MA, USA) according to the accompanying instructions. Using the ReverAce qPCR RT Master Mix with gDNA Remover (Toyobo, Osaka, Japan) kit, 1 microgram of total RNA was taken for the synthesis of the first strand of cDNA. Primer 5.00 (Palo Alto, CA) was used to design specific primers F, R (F: 5'-ATGAAGCCCTTCAGTTTGTC-3'; R: 5'-TCAATATTCGCAGCAAAAGG-3') to amplify the opening of HeHamp II-2 Reading Frame (ORF). In this study, the PCR reaction followed the following cycle parameters: 95°C for 5 min; 36 cycle...
Embodiment 2
[0023] Example 2 Biological activity detection of the mature peptide hepcidin (HeHampⅡ-2) in striae hippocampus
[0024] Synthesis of HeHampⅡ-2 Mature Peptide
[0025] The amino acid sequence of the mature peptide HeHampII-2 (63-86) is: HNRGCRFCCNCCGRTHFCAFCCEY, or as shown in SEQ ID NO:1. Using solid-phase peptide synthesis, it was synthesized by GenScript Biotechnology Company (Nanjing, China). The molecular mass and purity of the synthetic peptide were 2.834kD and 95.6%, respectively. Synthetic peptides were stored as dry powders at -80°C until use.
[0026] Microbial agglutination test
[0027] Gram-negative bacteria used in agglutination experiments: Vibrio parahaemolyticus, Vibrio harveta, Escherichia coli, multidrug-resistant Escherichia coli, Acinetobacter baumannii, drug-resistant strains of Acinetobacter baumannii, Klebsiella pneumoniae, Salmonella typhimurium; Gram-positive bacteria: Bacillus thuringiensis, Micrococcus luteus, Staphylococcus aureus, methicillin-...
Embodiment 3
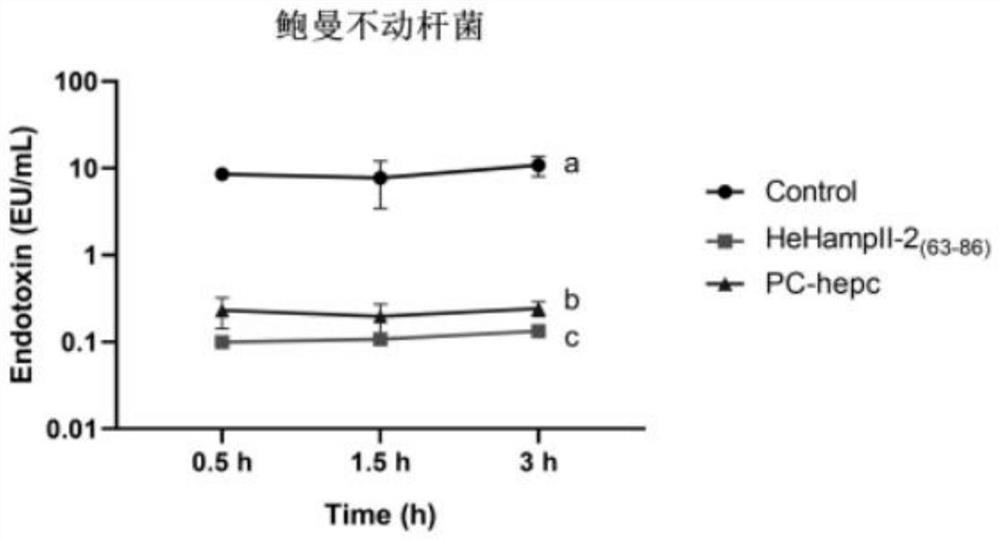
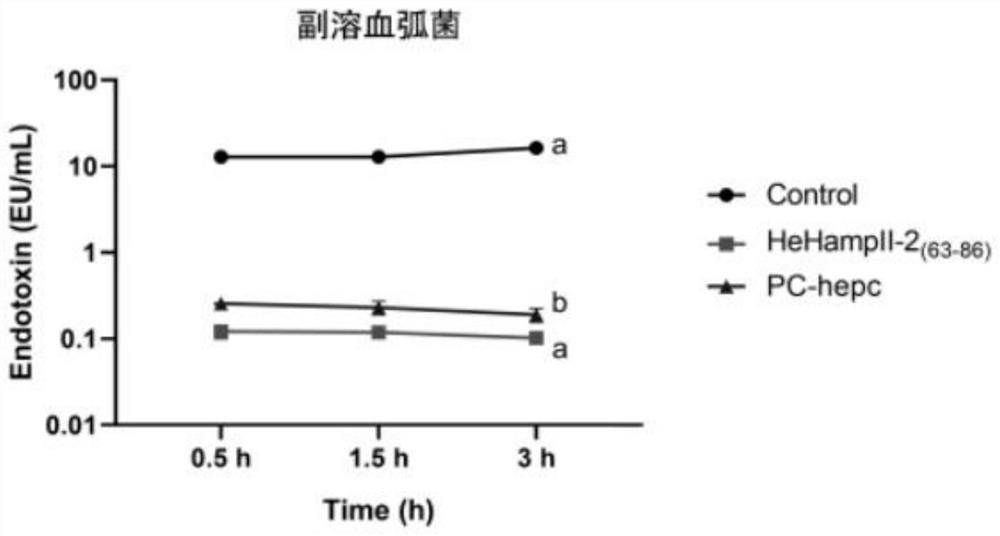
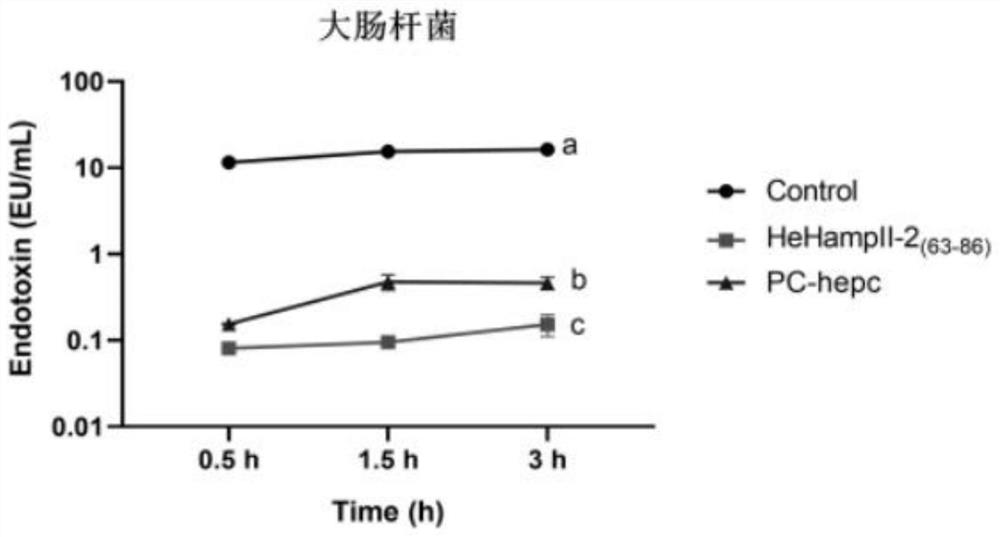
[0032] Example 3 Bacterial endotoxin inhibition experiment
[0033] The reagents and consumables used in this experiment are sterile and endotoxin-free.
[0034] Incubate Escherichia coli, Acinetobacter baumannii and Vibrio parahaemolyticus at the optimum temperature overnight, collect the cells, wash 3 times with 1×PBS, and resuspend to 1×10 with 1×PBS 5 cfu mL -1. The experimental group was as follows: the synthetic peptide was mixed with the bacterial solution in equal volume (when Escherichia coli and Vibrio parahaemolyticus were treated, the final concentration of protein in the mixture of protein and bacterial solution was 6 μM; when Acinetobacter baumannii was treated, the final concentration of protein and bacterial solution was 6 μM; The final concentration of protein was 6 μM in the liquid mixture; the control group was: protein solvent (DMSO solution with a volume ratio of 1 / 1000) was mixed with equal volume of bacterial solution, and the mature peptide of hepcidi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com