Single variable domain antibody targeting human programmed death ligand 1 (PD-L1) and derivative of single variable domain antibody
A PD-L1, domain antibody technology, applied in the direction of antibodies, anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc. In order to achieve the effect of inhibiting tumor growth, flexible application form, and good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Phage Display Camel Single Variable Domain Antibody Immune Library Construction
[0066] Antigens were used to immunize camels, peripheral blood mononuclear cells (PBMCs) were isolated and total RNA was extracted for reverse transcription, and the reverse transcription product was used as a template to amplify the variable domain of the heavy-chain antibody domain (variable domain of the heavy-chain) ofheavychain antibody, VHH) and connected to the phage display vector, electroporated into Escherichia coli TG1 competent cells, and camel immune library was constructed. Camels are immunized every two weeks, a total of 4 times. Each injection of 0.8mg PD-L1 extracellular region recombinant protein (self-expression and purification, gene sequence number: NP_054862.1, 19aa-238aa), supplemented with Freund's incomplete adjuvant (Sigma, Cat: F5506-10ml), take Subcutaneous multi-point injection. Two weeks after each immunization, 1 mL of serum was collected, and th...
Embodiment 2
[0067] Example 2: Anti-human PD-L1 specific single variable domain antibody screening
[0068] The constructed camel immune library was screened by solid-phase screening to obtain specific phage-displayed single variable domain antibodies.
[0069] (1) The original library is presented. The camel immune library was transferred to 2YT medium containing ampicillin and tetracycline, cultivated to the logarithmic growth phase, added M13 helper phage, and then added kanamycin, and presented overnight at a lower temperature. The culture supernatant was collected the next day, and the phages were concentrated by PEG precipitation to obtain high-titer antibody library display products for subsequent screening.
[0070] (2) Screening. Screening of specific antibodies was carried out by solid-phase method. Coat the specific antigen on the surface of the immune tube, block the immune tube and the antibody library with a blocking agent, add the antibody library to the immune tube and inc...
Embodiment 3
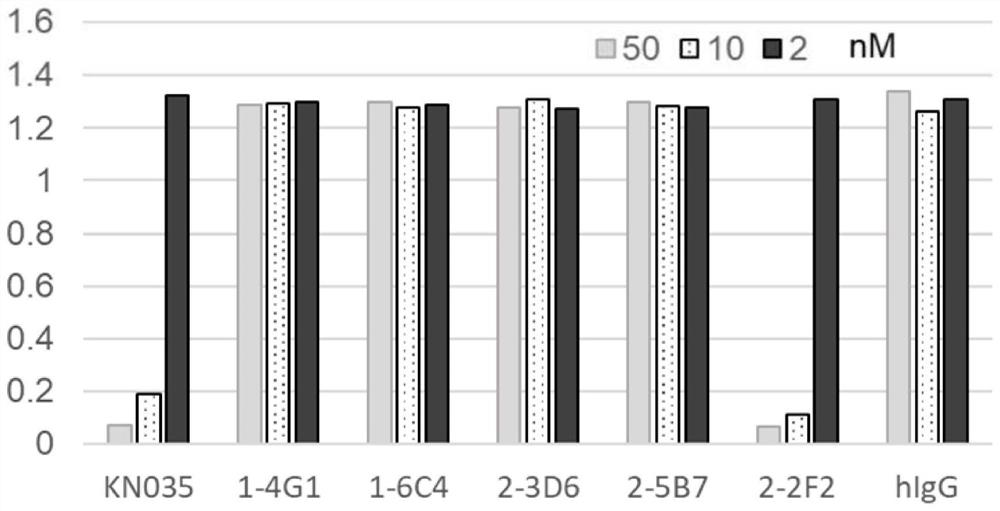
[0072] Example 3: Preliminary identification of anti-human PD-L1 specific single variable domain antibody
[0073] The five obtained single-domain VHH antibodies were induced and expressed in Escherichia coli TG1, and the induction conditions were 1 mM IPTG, 30°C, 150 rpm overnight culture. Bacterial samples with induced expression were sonicated and filtered, followed by affinity purification using a nickel column and ultrafiltration to obtain single-domain VHH antibodies. The inhibitory effect of the single domain on the binding of human PD-L1 to its receptor PD-1 was then detected by ELISA. Specifically, the fusion protein of human PD-1 extracellular region and human Fc (PD-1-hFc, PD-1 sequence number: NP_005009.2, 21aa-167aa) was coated at a concentration of 0.5 μg / mL overnight at 4°C. , blocked with 5% BSA for 60 min in a 37°C incubator. Single-domain VHH antibodies (concentrations of 50, 10, and 2 nM) were co-incubated with 1 μg / mL PD-L1-mFc, and reacted in a 37°C cons...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com