Application of heterocyclic compound containing at least two sulfur atoms to preparation of nano vaccine and prepared nano vaccine
A nano-vaccine and compound technology, applied in medical preparations containing active ingredients, medical preparations with non-active ingredients, nanotechnology, etc., can solve the problem of reducing cross-presentation of antigens, inability to avoid enzymatic degradation in lysosomes, Reduce stimulation and activation-promoting effects, achieve high-efficiency immune activation and immune regulation, prevent tumor cell proliferation and virus infection, and improve cross-presentation effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Compound 1: Synthesized in Example 1;
Embodiment 2
[0081] Compound 2: Synthesized in Example 2;
[0082] Compound 3: purchased from McLean Biochemical Technology Co., Ltd.
[0083] Below in conjunction with embodiment, further set forth the present invention:
[0084] Example 1
[0085] The dichloromethane solution containing N,N'-carbonyldiimidazole and lipoic acid was gradually dropped into the dichloromethane of ethylenediamine at 0°C, stirred at 0°C for 1h and at room temperature for another 1h, and washed with anhydrous Sodium sulfate was used to remove water, and then concentrated under reduced pressure to obtain an oily substance. Dissolve the oil in a dichloromethane solution containing 1H-pyrazole-1-carboxamidine hydrochloride, stir, distill under reduced pressure, dissolve the obtained precipitate in methanol, wash the precipitate with ether, and finally obtain Compound 1.
[0086]
[0087] Example 2
[0088]
[0089] Molecule 1 of anhydrous CH 2 Cl 2 Add 1,1-carbonyldiimidazole (CDI) to the solution...
Embodiment 3
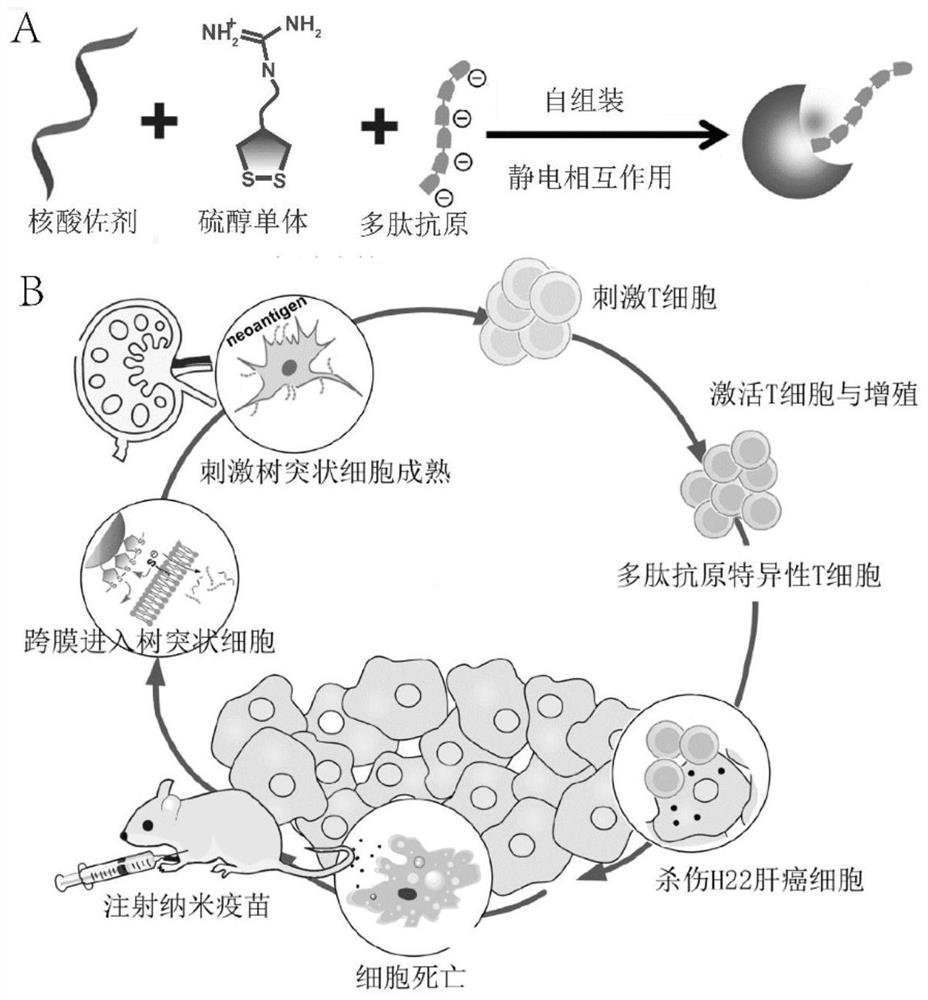
[0091] Nano vaccine preparation method: the compound 1 (concentration is 146.2mM) that embodiment 1 makes, the nucleic acid adjuvant (CpG-ODN) of 100mM, the polypeptide antigen of 2.5mg / mL (mouse liver cancer cell H22 polypeptide antigen, amino acid sequence It is HTDAHAQAFAALFDSMH, the N-terminal is connected with Cy3 label, and the isoelectric point is 5.71) mixed according to the volume ratio of 1:1:1, and put into the TM buffer buffer solution (pH 7.4) of 60-70 times the total volume, at 37 Mix and stir for 15 minutes at ℃, and dialyze in deionized water for 24 hours to remove unloaded nucleic acid adjuvant or polypeptide antigen, and finally obtain the nano-vaccine.
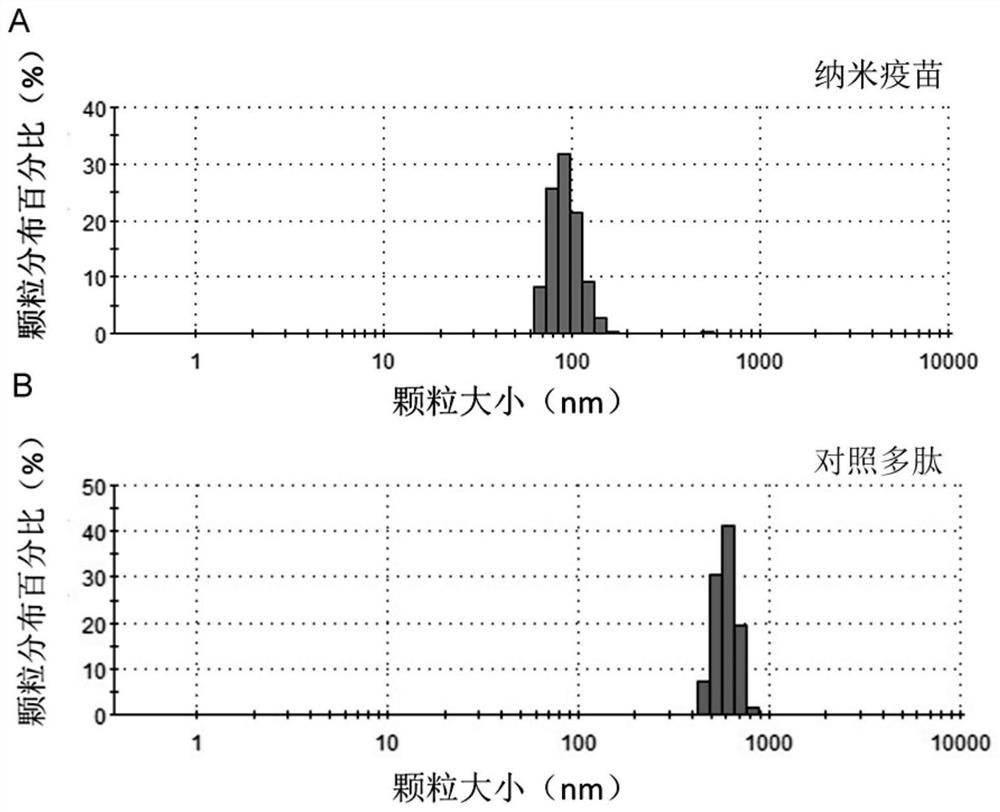
[0092] After the nano-vaccine was prepared by shock dialysis, the particle size was measured and the scanning electron microscope was used to characterize the successful preparation of the nano-vaccine. The results show that CpG-ODN, polypeptide antigen and compound 1 can form a spherical nano-vaccine in TM ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com