Method for grafting dialkyl phosphinic acid functional group on surface of silicon-based material
An alkyl phosphinic acid functional, monoalkyl phosphinic acid technology, applied in chemical instruments and methods, alkali metal oxides/hydroxides, inorganic chemistry, etc., can solve the problem of poor stability and cycle performance of leaching resins , the extraction agent cannot be effectively used, the extraction agent is lost, etc., to achieve the effect of good recycling performance, fast adsorption speed, and fast adsorption kinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
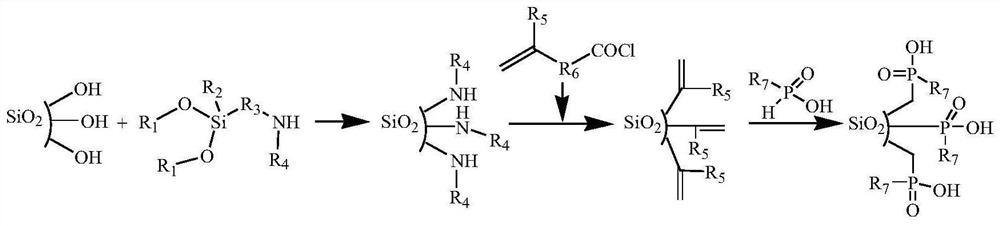
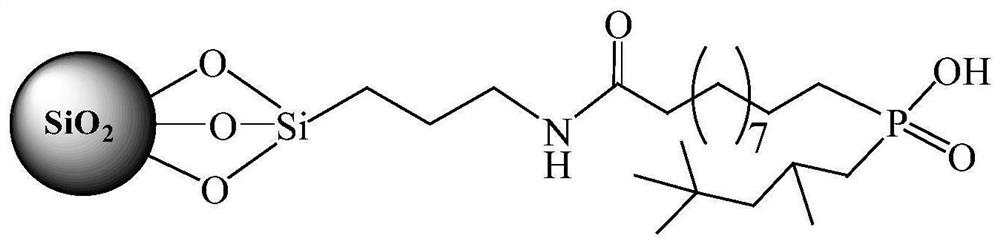
[0030] (1) Silica gel activation: Reflux 100g of 60-80mesh silica gel particles in 180mL 6mol / L HCl for 6h, cool down to room temperature, filter, wash repeatedly with deionized water, and dry at 120°C for 6h to obtain activated silica gel particles.
[0031] (2) Grafting amino groups on the surface of silica gel: 20 mL of 3-aminopropyltriethoxysilane and 200 mL of toluene were added to 20 g of activated silica gel, and the mixture was refluxed for 6 h. After cooling down to room temperature, the supernatant was removed by centrifugation, and the silica gel particles were washed twice with ethanol and twice with acetone, and air-dried at room temperature to obtain silica gel particles grafted with amino groups on the surface.
[0032] (3) Preparation of acid chlorides containing C=C double bonds: add 7.48g thionyl chloride to 10.14g undecylenic acid, react at 60°C for 4h, then react at 80°C for half an hour, drop to room temperature, pass The excess thionyl chloride was remove...
Embodiment 2
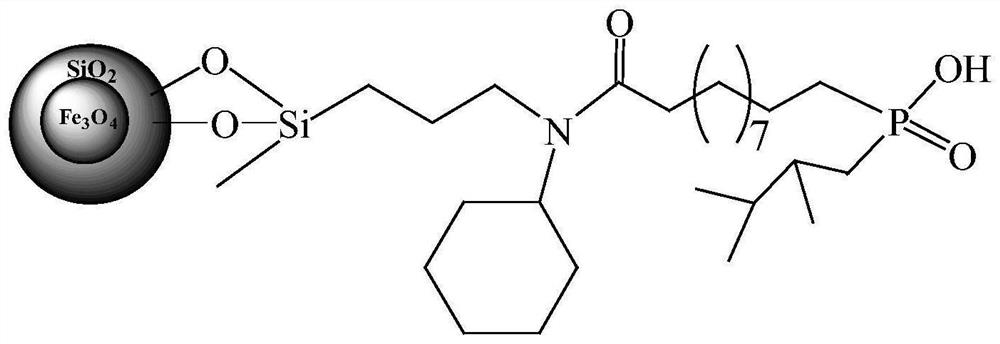
[0036] (1) Fe 3 o 4 @SiO 2 Amino groups grafted on the surface of magnetic microspheres: to 20g Fe 3 o 4 @SiO 210 mL of N-cyclohexyl-γ-aminopropylmethyldimethoxysilane and 180 mL of 1,4-dioxane were added to the magnetic microspheres, and the mixture was refluxed for 10 h. After cooling down to room temperature, the supernatant was removed by centrifugation, washed twice with ethanol and twice with acetone, and air-dried at room temperature to obtain Fe with amino groups grafted on the surface. 3 o 4 @SiO 2 magnetic microspheres.
[0037] (2) Fe 3 o 4 @SiO 2 C=C double bond modification on the surface of magnetic microspheres: take 18g of amino-modified Fe 3 o 4 @SiO 2 Add 50mL tetrahydrofuran and 8.6g anhydrous sodium carbonate to the magnetic microspheres, slowly add the tetrahydrofuran solution of undecanoyl chloride through a constant pressure funnel at room temperature, and react for 3 hours after the dropwise addition. Centrifuge, remove supernatant, Fe 3 ...
Embodiment 3
[0040] (1) Amino groups grafted on the surface of SBA-15: Add 15mL N-(β-aminoethyl)-γ-aminopropylmethyl-dimethoxysilane and 150mL 1,4-dioxosilane to 12g SBA-15 Six rings, reflux reaction for 24h. After cooling down to room temperature, the supernatant was removed by centrifugation, washed three times with ethanol and three times with acetone, and air-dried at room temperature to obtain SBA-15 with amino groups grafted on the surface.
[0041] (2) Preparation of acid chlorides containing C=C double bonds: add 4.8g thionyl chloride to 4.2g 5-hexenoic acid, react for 2 hours at 80°C, drop to room temperature, remove excess chlorine by rotary evaporator Sulfoxide was added, and 10 mL of 1,4-dioxane was added to obtain an organic solution of 5-hexenoyl chloride.
[0042] (3) C=C double bond modification on the surface of SBA-15: Take 12g amino-modified SBA-15, add 50mL 1,4-dioxane and 5.5g sodium hydroxide, and slowly add it through a constant pressure funnel at room temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com