Synthesis method of 5-bromo-1, 2, 3-trimethoxybenzene
A technology of trimethoxybenzene and synthesis method, applied in chemical instruments and methods, preparation of organic compounds, preparation of ethers by ester reaction, etc., can solve problems such as difficulty, not cheap, not economical, etc., and achieve cheap and easy to obtain raw materials, The effect of good safety and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
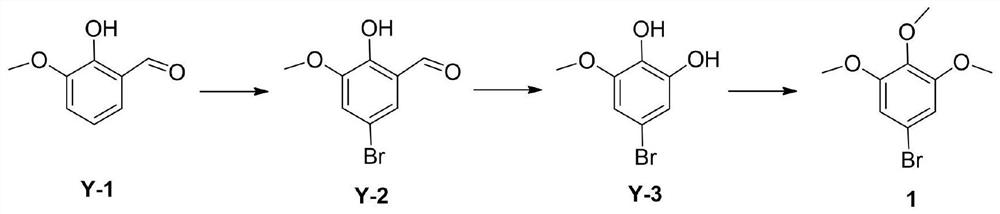
[0026] According to an embodiment of the present invention combined with figure 1 show. A kind of synthetic method of 5-bromo-1,2,3-trimethoxybenzene, comprising the steps:
[0027] S1. Dissolve Linvanillin in the solvent and lower the temperature to 10-20°C, add the acid-binding agent to the system to promote the reaction speed and complete reaction, and control the temperature of the system not higher than 20°C. Add brominating agent to the system in batches and stir until the reaction is complete, filter, wash and dry to obtain 5-bromo-2-hydroxy-3-methoxybenzaldehyde;
[0028] S2. Dissolve the product obtained in S1 in a solvent, and add an oxidizing agent to the system in batches under an environment where the system temperature does not exceed 40°C until the reaction is completed. After separation, washing and drying, 5-bromo-3-methoxybenzene is obtained. -1,2-diphenol;
[0029] S3. Dissolve the product obtained in S2 in a solvent and add a catalyst to the system, rais...
Embodiment 1
[0039] Synthesis of S1, 5-bromo-2-hydroxyl-3-methoxybenzaldehyde (Y-2)
[0040] Dissolve 500 grams of o-vanillin (Y-1) in 7 liters of glacial acetic acid, cool down to 15 degrees, add sodium acetate, the temperature rises significantly, continue to cool down to 15 degrees, stir for 30 minutes, add liquid bromine dropwise, and control the temperature for 20 minutes. below the degree. After adding about 75% liquid bromine dropwise, a yellow solid will precipitate and the system will become viscous. After the dropwise addition, the stirring was continued for 1.5 to 2.0 hours until the reaction was complete. The reaction solution was cooled to 15°C, poured into 3 liters of ice water and stirred for 25 minutes, then filtered, and the filter cake was washed with 500 ml of ice water and an appropriate amount of ethanol. Blast drying at 45 degrees to obtain about 760 grams of Y-2.
[0041] Synthesis of S2, 5-bromo-3-methoxybenzene-1,2-diphenol (Y-3)
[0042] Dissolve 1 kg of Y-2 i...
Embodiment 2
[0046] The brominating agent in S1 is N-bromosuccinimide, the acid-binding agent is sodium carbonate; the oxidant in S2 is calcium carbonate; the catalyst in S3 is potassium carbonate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com