An m-ti hydrotalcite-based closed boron cluster mb x h x Preparation method and application of nanometer precious metal
A closed-type, hydrotalcite-based technology, which is applied in the field of preparation of M-Ti hydrotalcite-based closed-type boron cluster MBxHx nanoscale noble metals, can solve the problems of difficult catalyst recovery, high reaction temperature, and unfriendly environment, and achieve reuse High rate, high conversion rate, good environmental effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Dissolve 4.0003g of urea in 100mL of water, and add 0.2003g of TiCl dropwise simultaneously 4 and 1.0011g closed boron cluster MgB 12 H 12 solution;
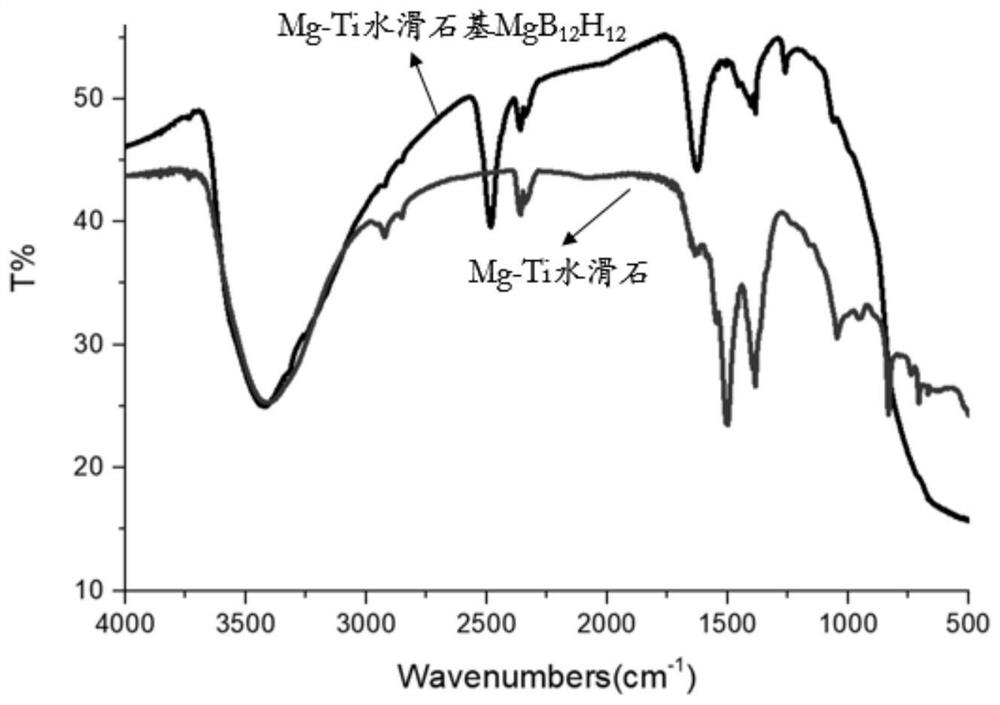
[0029] (2) Continue stirring for 3h under nitrogen protection, age in a hydrothermal kettle for 24h at 140°C, filter, wash and dry to obtain 2.8429g Mg-Ti hydrotalcite-based closed boron cluster MgB 12 H 12 ,like figure 1 As shown, the Mg-Ti hydrotalcite-based closed boron cluster MgB prepared in this example 12 H 12 The infrared spectrum of , the successful combination of hydrotalcite and boron clusters can be seen by Fourier transform infrared spectroscopy (FT-IR);
[0030] (3) 2.8001Mg-Ti hydrotalcite-based closed boron cluster MgB 12 H 12 Disperse in 50 mL of water, add 0.0558 g of sodium chloroaurate, stir and react for 5 min at 20 °C under a 365 nm UV lamp, filter to obtain a precipitate, repeatedly wash with ethanol and water, and dry at room temperature to obtain 2.8357 g of Mg-Ti hydrotalcite-based co...
Embodiment 2
[0033] (1) Dissolve 3.7416g of urea in 100mL of water, and add 0.2481g of TiCl dropwise simultaneously 4 and 1.2469g closed boron cluster CoB 12 H 12 solution;
[0034] (2) Continue stirring for 1 h under nitrogen protection, age in a hydrothermal kettle for 48 h at 120 °C, filter, wash and dry to obtain 3.2094 g Co-Ti hydrotalcite-based closed boron cluster CoB 12 H 12 ;
[0035] (3) 3.2001g Co-Ti hydrotalcite-based closed boron cluster CoB 12 H 12 Disperse in 50mL of water, add 0.0798g of sodium chloropalladate, stir and react for 10min at 35°C under a 400nm UV lamp, filter to obtain a precipitate, repeatedly wash with ethanol and water, and dry at room temperature to obtain 3.2518g of Co-Ti hydrotalcite base compound. boron cluster CoB 12 H 12 Nano-palladium catalyst;
[0036] (4) Dissolve 4.0102g of benzene in 10mL of acetonitrile, add 0.2004g of Co-Ti hydrotalcite-based closed boron cluster CoB 12 H 12 The nano-palladium catalyst and 3.9746g of 30% hydrogen per...
Embodiment 3
[0038] (1) Dissolve 2.8614g NaOH in 100mL water, and add 0.1910g butyl phthalate and 0.7628g closed boron cluster MgB dropwise at the same time. 10 H 10 solution;
[0039] (2) Continue stirring for 2h under nitrogen protection, age in a hydrothermal kettle for 72h at 80°C, filter, wash and dry to obtain 2.1778g Mg-Ti hydrotalcite-based closed boron cluster MgB 10 H 10 ;
[0040] (3) 2.0005g Mg-Ti hydrotalcite-based closed boron cluster MgB 10 H 10 Disperse in 50 mL of water, add 0.0399 g of sodium chloroplatinate, stir and react for 10 min at 40 °C under a 200 nm UV lamp, filter to obtain a precipitate, repeatedly wash with ethanol and water, and dry at room temperature to obtain 2.0341 g of Mg-Ti hydrotalcite-based compound. boron cluster MgB 10 H 10 Nano platinum catalyst;
[0041] (4) Dissolve 1.9989g benzene in 10mL acetonitrile, add 0.1975g Mg-Ti hydrotalcite-based closed boron cluster MgB 10 H 10 Nano-platinum catalyst and 2.9891g of 30% hydrogen peroxide were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com