Preparation method of 5-aminolevulinic acid hydrochloride
A technique for the synthesis of aminolevulinic acid hydrochloride and its synthesis method, which is applied in the field of synthesis of drugs and drug intermediates, can solve the problems of expensive raw materials, easy pollution, long synthesis route, etc., and achieve high product yield, convenient operation, Quality Controlled Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
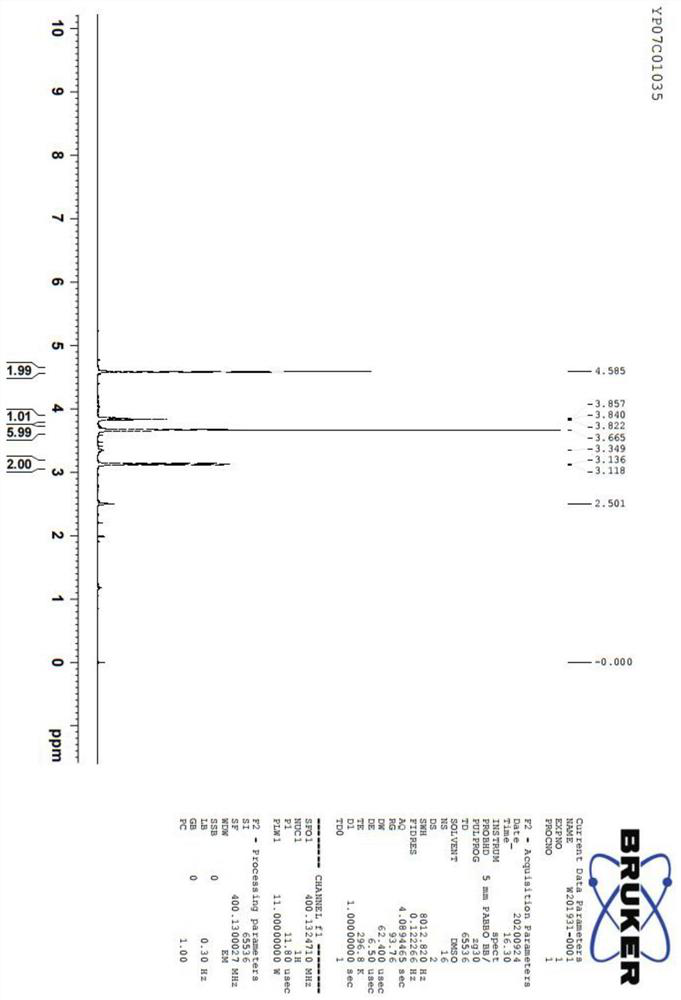
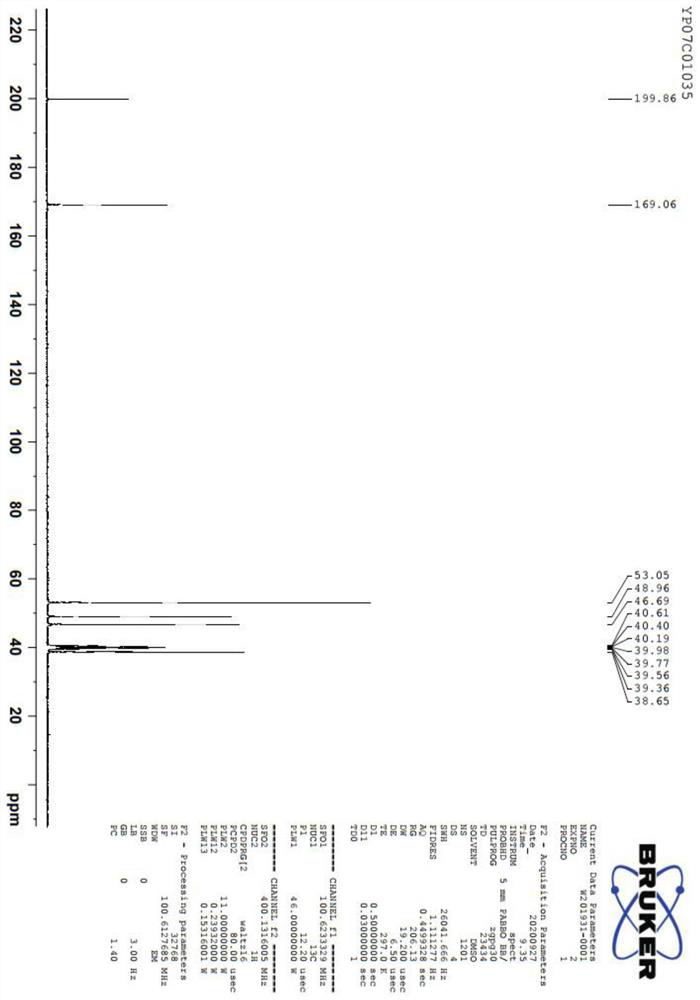
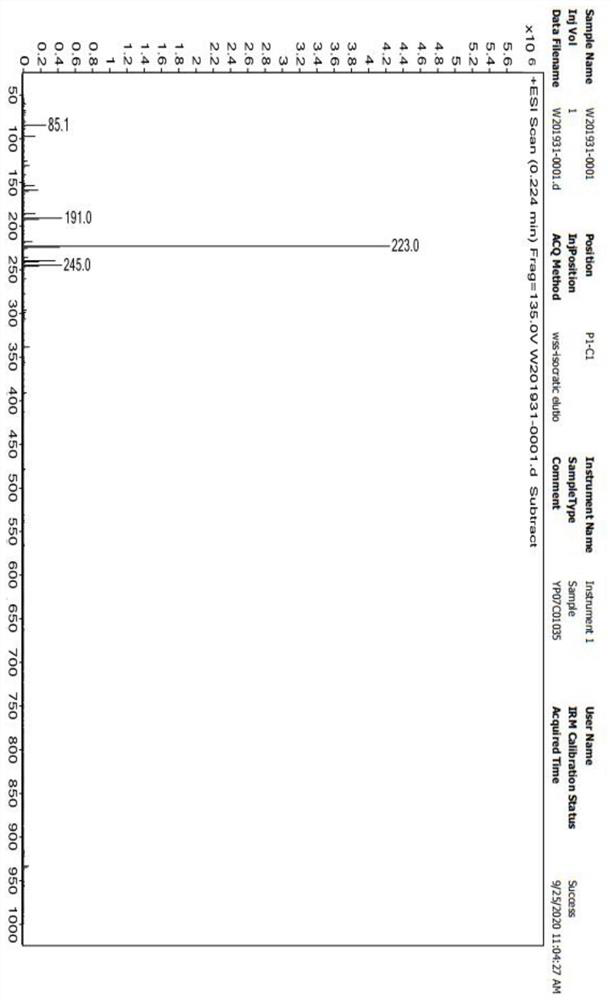
Image
Examples
Embodiment 1
[0044] A preparation method of 5-aminolevulinic acid hydrochloride, comprising the following steps:
[0045] (1) Preparation of 2-(3-chloro-2-oxypropyl) dimethyl malonate
[0046] Add 16.51 g of 1,3-dichloroacetone and 100 ml of methanol into a 500 ml three-necked flask, and stir magnetically. 13.21g of dimethyl malonate and 20ml of triethylamine dissolved in 50ml of methanol were slowly added dropwise to the 1,3-dichloroacetone solution, and the drop was completed in half an hour. Stir at room temperature for 4h. After concentrating under reduced pressure to dryness, add 100ml of dichloromethane, wash with purified water 3 times, each time with 50ml of water, and concentrate under reduced pressure to obtain 20.50g of colorless liquid (intermediate I). The yield of this step is 92%, and the purity is 96%. .
[0047] (2) Preparation of dimethyl 2-(3-(1,3-dioxoisoindol-2-yl)-2-oxopropyl)malonate
[0048] Add 11.13g of intermediate I, 12.03g of potassium imide phthalate and 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com