Esterase mutant Est8-XL with improved activity and application thereof
A technology of est8-xl and mutants, applied to esterase mutant Est8-XL and its application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Construction of embodiment 1 mutant library
[0034] The esterase gene est8 in the thermophilic Bacillus sp. K91 strain was amplified by error-prone PCR to obtain the mutant sequence. Error-prone PCR was performed according to the instructions of the GeneMorph II EZClone Domain Mutagenesis Kit Random Mutagenesis Kit (purchased from Agilent Technologies Co., Ltd.).
[0035] 1) Est8 mutation large primer PCR
[0036] Using the recombinant plasmid containing the gene est8 extracted from the E.coliBL21(DE3) expression recombinant strain as the template for PCR, design primers:
[0037] Upstream primer est8F: 5'-GCAAATCATATTTATCTTGC-3';
[0038] Downstream primer est8R: 5'-CCTTTCTTTGATGATCGATTC-3'.
[0039] The PCR reaction system is as follows:
[0040]
[0041]
[0042] PCR reaction parameters: pre-denaturation at 95°C for 5 minutes; then denaturation at 95°C for 30 sec, annealing at 42°C for 30 sec, extension at 72°C for 1 min, and incubation at 72°C for 10 min ...
Embodiment 2
[0058] Example 2 Screening of mutants with improved deacetylation activity
[0059] 1) Primary screening: 480 mutants were screened.
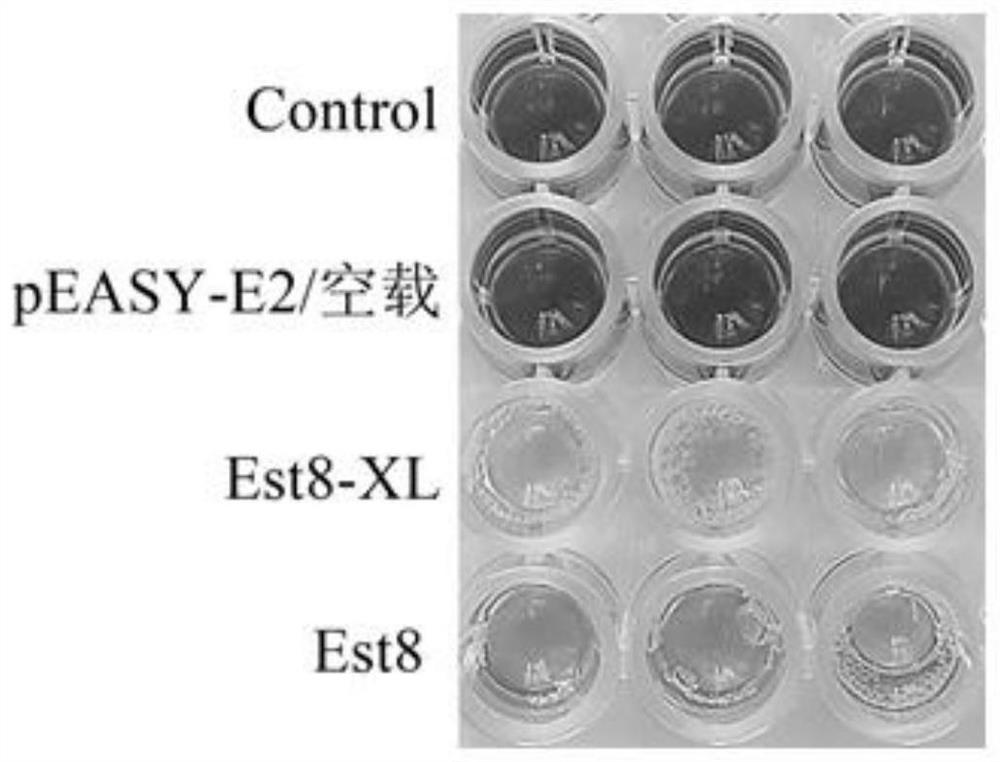
[0060] Add 100 μL of substrate / well (the substrate is 50 mM 7-ACA, 0.02% BTB, and 50 mM phosphate buffer at pH 7.3) to 96-well ELISA plate; add 50 μL of cell lysate / well to 96 After mixing in the microplate plate, react at 25°C for 10 minutes, and then detect the OD value under a microplate reader with an absorbance of 616nm. Here, the colorimetric method is used to screen mutants: since esterase can catalyze 7-ACA to generate D-7-ACA and acetic acid, the pH of the solution drops at this time, and when there is an indicator of 0.02% BTB, the solution will turn from blue to blue. Green and then yellow, the more yellow the solution, the higher the activity of the enzyme.
[0061] 2) Twice re-screening: screen the 40 mutants screened out initially
[0062] The screened 40 mutants with high 7-ACA deacetylation activity were picked on a 96-well p...
Embodiment 3
[0064] Embodiment 3 preparation of wild enzyme Est8 and mutant Est8-XL
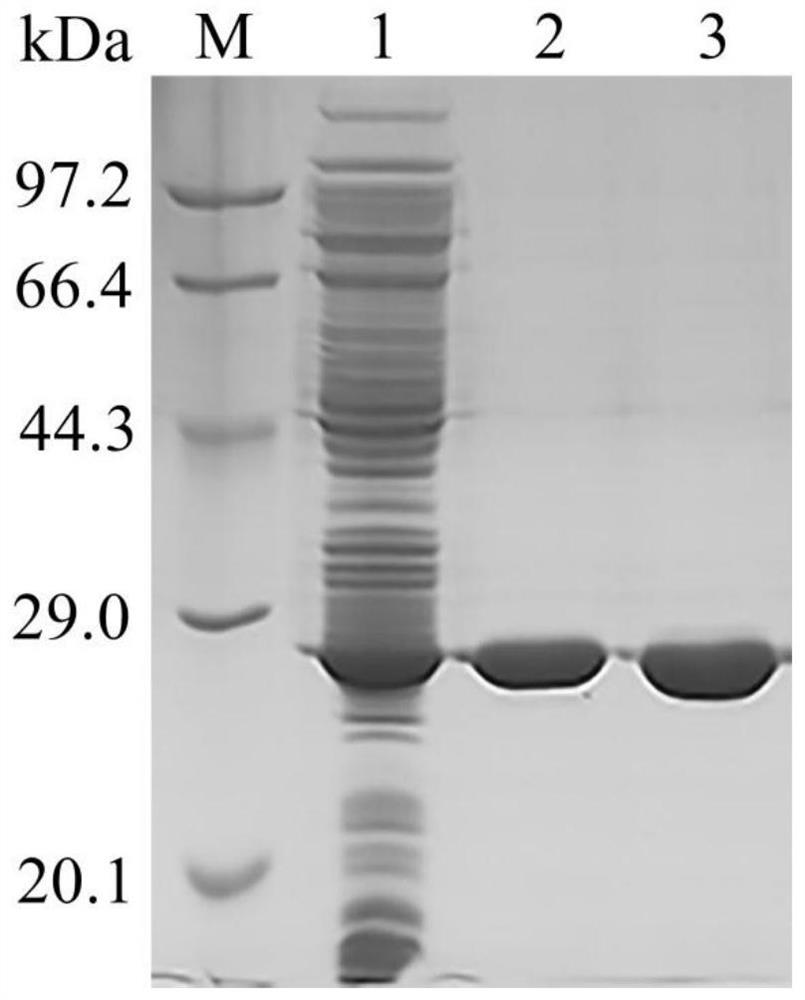
[0065] The recombinant strains containing the wild enzyme Est8 and the mutant Est8-XL were added to the test tubes containing 5 mL of Amp-LB liquid medium at an inoculation amount of 1‰ respectively (37 ° C, 180 rpm); after 15 hours, 4 mL was added to 400 mL Cultivate in Amp-LB liquid medium for 2.5h; add Isopropylβ-D-Thiogalactoside (IPTG) at a final concentration of 1.4mM to induce for 21h at 20°C and 150rpm; use a refrigerated centrifuge Collect the bacteria (4°C, 5000rpm, 10min); resuspend the bacteria with Tris-HCl 8.0 buffer and break the cells with an ultrasonic cell crusher; centrifuge at 12000rpm, 4°C for 20min to collect the supernatant; The supernatant protein solution was added to a Nickel-NTAAgarose purification column and eluted with a gradient elution solution containing 0-500mM imidazole to purify the target protein.
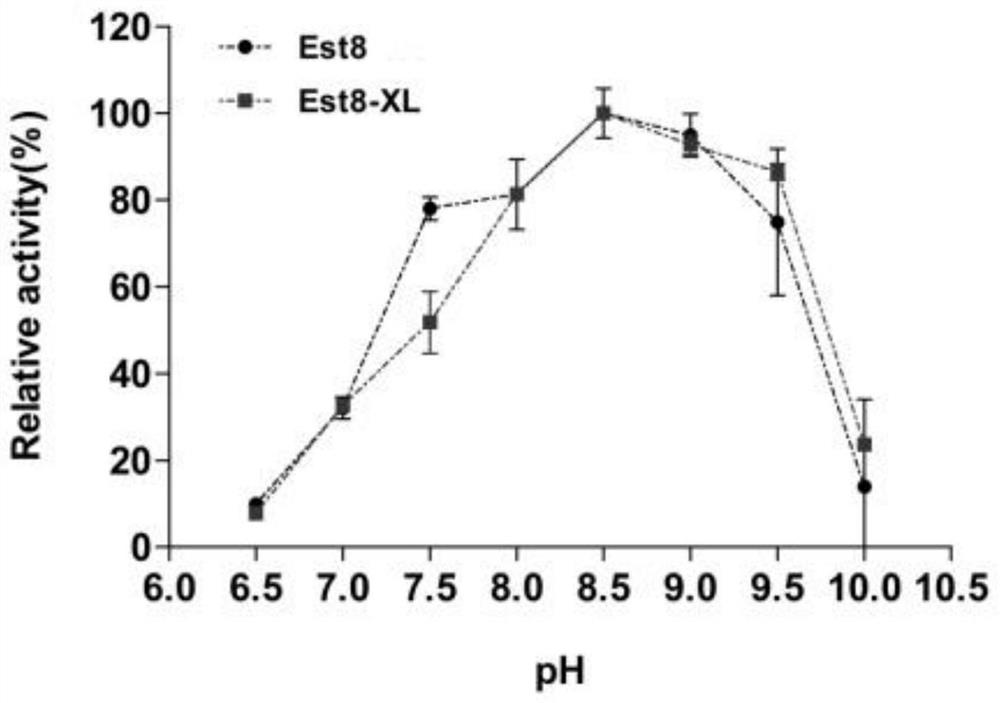
[0066] The SDS-PAGE results showed that both the mutant enzyme Est8-XL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com