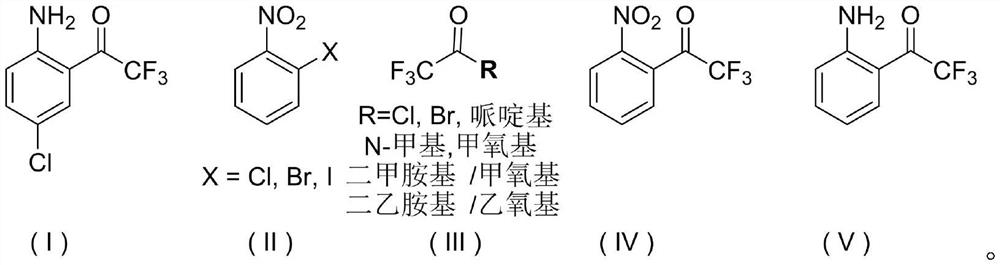
Synthetic method of efavirenz intermediate namely 1-(2-amino-5-chlorphenyl)-2, 2, 2-trifluoroethanone
A technology of efavirenz and trifluoroethyl ketone, which is applied in the field of synthesis of pharmaceutical and chemical intermediates, can solve the problems of isopropylmagnesium bromide being expensive, not meeting the requirements of green chemistry, and difficult reaction conditions, so as to reduce the generation of three wastes Quantity, short steps, and the effect of shortening the reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of 1-(2-nitrophenyl)-2,2,2-trifluoroethanone (IV)
[0040] Take a 500mL four-necked bottle equipped with a condenser, a mechanical stirrer, a dropping funnel, and a thermometer, add 7.6g (320mmol) of magnesium chips, 1.79g of initiator bromobenzene (11mmol), and 52mL of tetrahydrofuran. Bromobenzene (40.8g, 260mmol diluted with 180mL tetrahydrofuran) was added dropwise under reflux state, and the dropwise addition time was 1-2h, and the reflux reaction was continued for 4h after dropping to obtain phenylmagnesium bromide Grignard reagent. The preparation method of the invented phenylmagnesium chloride Grignard reagent is the same as that of the above phenylmagnesium bromide Grignard reagent; after the reaction system is cooled to -20°C, o-iodonitrobenzene (200mmol, 49.8g) is dissolved in 40mL of tetrahydrofuran solution and added dropwise During the dropwise addition, the temperature should not exceed -10°C. After the dropwise reaction was complet...
Embodiment 2
[0043] Example 2: Preparation of 1-(2-aminophenyl)-2,2,2-trifluoroethanone (V)
[0044] Take 500mL autoclave, add 1-(2-nitrophenyl)-2,2,2-trifluoroethanone (100mmol, 21.9g), 95% ethanol (140mL), Raney Ni catalyst (1.3g) successively , feed hydrogen, pressure to 0.6-0.7MPa, heat up to 50-60°C and keep stirring, wait until the reaction is complete, filter and recover the catalyst, concentrate the organic phase under reduced pressure to recover ethanol to dryness, and recrystallize the residue with n-octanol (60mL) Compound (V) was obtained as a dark yellow solid (17.4 g, yield 92%) with a melting point of 51-52°C.
[0045] Spectrum characterization of compound (Ⅳ):
[0046] 1 H NMR (400MHz, CDCl 3 )δ=7.75(d, J=8.4Hz, 1H), 7.38(t, J=7.8Hz, 1H), 6.71(dd, J=13.8, 8.2Hz, 2H), 6.41(s, 2H). 13 C NMR (100MHz, CDCl 3 )δ=180.8(q, J C-F =30Hz), 153.1, 136.6, 131.3, 117.4, 117.0 (q, J C-F =289Hz), 116.3, 111.0, 77.3, 77.0, 76.7.
Embodiment 3
[0047] Example 3: Preparation of 1-(2-amino-5-chlorophenyl)-2,2,2-trifluoroethanone (I)
[0048] Take a 500mL two-necked round bottom reaction flask, add 1-(2-aminophenyl)-2,2,2-trifluoroethanone (100mmol, 19.2g), N-chlorosuccinimide (15.8g, 120mmol ), chloroform 150mL, catalyst dimethyl sulfoxide (0.71g, 10mmol), stirring at room temperature, until the reaction was complete, concentrated under reduced pressure to recover the solvent, and the residue was recrystallized from n-hexane (80mL) to obtain compound (I) ( 19.3 g, yield 86%), the product is a bright yellow solid with a melting point of 90-91°C and a purity of 98.5% as measured by HPLC (the mobile phase is acetonitrile:water=70:30, V / V).
[0049] Spectrum characterization of compound (I):
[0050] 1 H NMR (400MHz, DMSO-d 6 )δ=7.86(s,2H),7.45(d,J=2.8Hz,2H),6.98(d,J=8.4Hz,1H). 19 F NMR (376MHz, DMSO-d 6 )δ=-68.90,-83.12. 13 C NMR (100MHz, DMSO-d 6 )δ=178.2(q, J C-F =30Hz), 153.7, 137.2, 128.6, 120.7, 118.6, 117.2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com