High-yield green and safe synthesis method of 3, 3', 4, 4'-biphenyltetracarboxylic dianhydride
A technology of biphenyltetracarboxylic dianhydride and biphenyltetracarboxylic acid, which is applied in the field of synthesis of acid anhydride raw material monomers of polyimide, can solve the problems of high cost, low synthesis technology yield, non-green environmental protection and the like, and achieves low pollution. , high yield, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
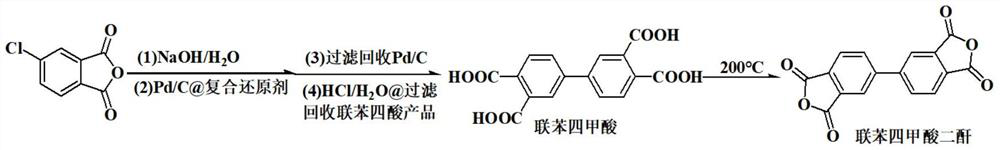
[0032] This example provides a high-yield green and safe synthesis method of 3,3',4,4'-biphenyltetracarboxylic dianhydride, the reaction equation of which is as follows:
[0033]
[0034] This synthetic method comprises the steps:
[0035] Add 400.0g of distilled water in the four-necked flask equipped with thermometer, stirrer, air duct and reflux device, add 27g of sodium hydroxide (0.675mol) under stirring, add 50g of 4-chlorophthalic anhydride (0.275mol) after dissolving ), stirred at room temperature for 1 hour, then added 0.3g of 10wt%Pd / C (with 0.5% concentration of glucose aqueous solution in 300 ℃ of nitrogen atmosphere to do the anhydrous palladium carbon of carbonization bonding treatment), 20g composite reducing agent (β-cyclodextrin / serine / xylitol=5 / 3 / 2), bubbling with nitrogen for 30min, then heating up to 95°C under nitrogen protection, reacting for 10h, cooling to room temperature, filtering, drying and drying under nitrogen protection Reclaim palladium-car...
Embodiment 2
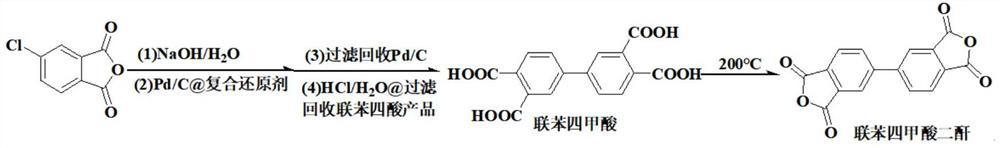
[0037] This example provides a high-yield green and safe synthesis method of 3,3',4,4'-biphenyltetracarboxylic dianhydride, the reaction equation of which is as follows:
[0038]
[0039] This synthetic method comprises the steps:
[0040] Add 400.0g of distilled water in the four-necked flask equipped with thermometer, stirrer, air duct and reflux device, add 27g of sodium hydroxide (0.675mol) under stirring, add 50g of 4-chlorophthalic anhydride (0.275mol) after dissolving ), stirred at room temperature for 1 hour, then added 0.306g of reclaimed 10%Pd / C from Example 1, 20g composite reducing agent (β-cyclodextrin / serine / xylitol=5 / 3 / 2) , nitrogen bubbling for 30min, then heated up to 95°C under nitrogen protection, reacted for 10h, cooled to room temperature and filtered, dried under nitrogen protection and recovered palladium carbon catalyst 0.305g (recovery rate 102%); 20% was added dropwise to the filtrate Concentrated hydrochloric acid to adjust the acidity to PH = 3;...
Embodiment 3
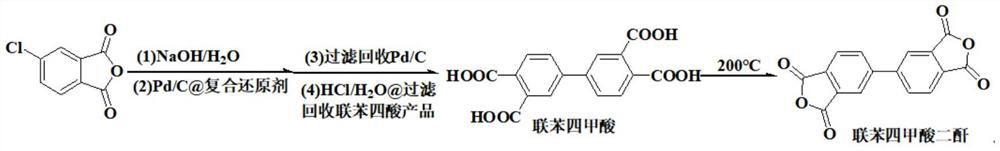
[0042] This example provides a high-yield green and safe synthesis method of 3,3',4,4'-biphenyltetracarboxylic dianhydride, the reaction equation of which is as follows:
[0043]
[0044] This synthetic method comprises the steps:
[0045] Add 400.0 g of distilled water to a four-neck flask equipped with a thermometer, a stirrer, an air duct and a reflux device, add 27 g of sodium hydroxide (0.675 mol) under stirring, and add 50 g of 4-chlorophthalic anhydride (0.275 mol) after dissolving , stirred at room temperature for 1 hour, then added 0.305g of 10wt% Pd / C recovered from Example 2, 40g composite reducing agent (-cyclodextrin / serine / xylitol=5 / 3 / 2), Nitrogen was bubbled for 30 min, then the temperature was raised to 95° C. under the protection of nitrogen, reacted for 10 h, cooled to room temperature and filtered, dried and recovered palladium carbon catalyst 0.304 g (recovery rate 101.3%) under the protection of nitrogen; Adjust the acidity with hydrochloric acid to PH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com