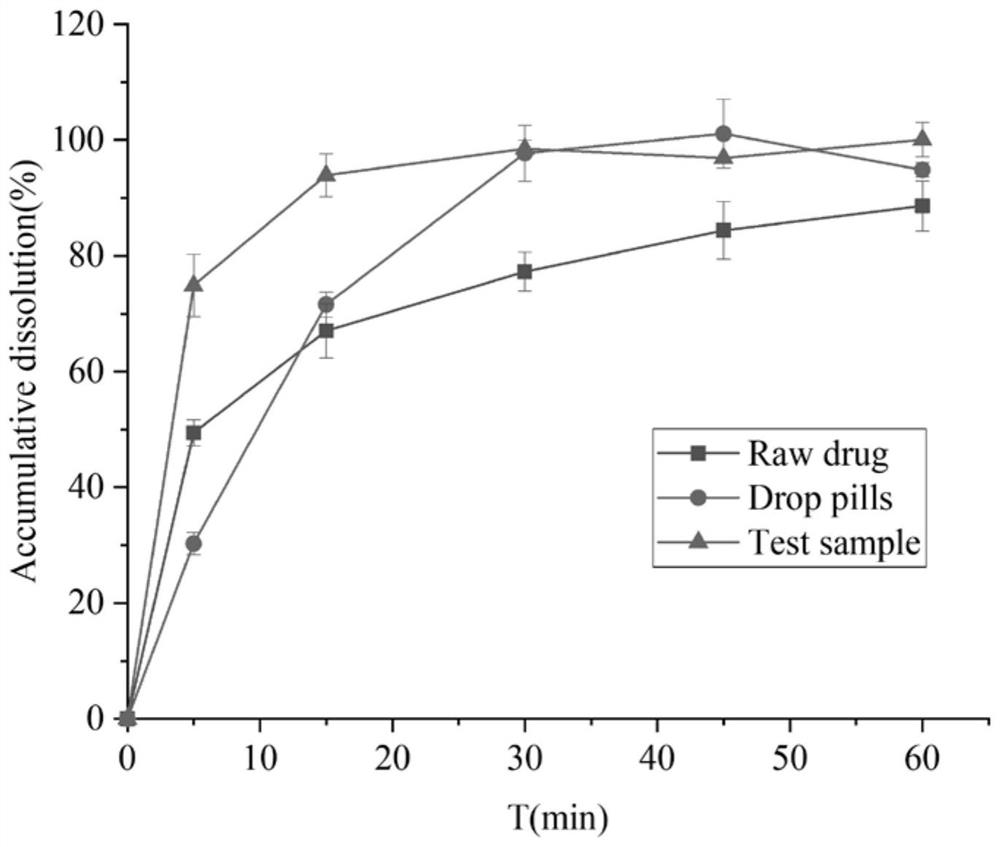
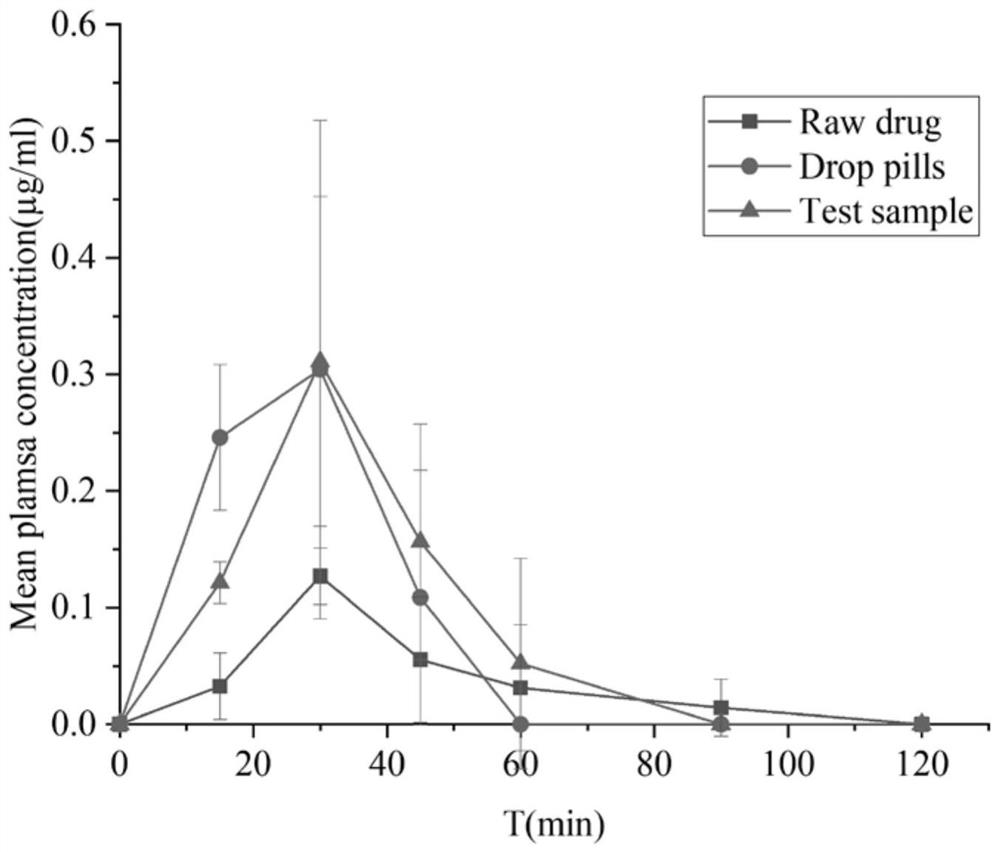
Solidified andrographolide solution for oral administration, and preparation method of solidified andrographolide solution
A solidification technology of andrographolide, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of changing the pharmacokinetic characteristics, tissue distribution characteristics, and crystallization of the main drug. Or crystallization coarsening, irritating gastrointestinal mucosa, etc., to achieve the effect of simple and easy preparation method, improved stability and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0041] Solubility Determination of Andrographolide in Different Solvents
[0042] Add andrographolide to PEG 200, PEG 400, PEG 600, Transcutol P, 1,2-propanediol, absolute ethanol, NMP, dimethyl sulfoxide, and dimethylformamide respectively, and grind in a mortar for 15 minutes , divided into two EP tubes, respectively placed in 25°C and 50°C collector-type constant temperature heating magnetic stirrers for 24h, centrifuged at 2000rpm for 10min, took 5 drops of the supernatant and weighed them accurately, and diluted them with methanol properly. Amount of 10 μL was injected into a high-performance liquid chromatograph for determination, the peak area was recorded, and the solubility of andrographolide in different solvents was calculated. The details are shown in Table 1.
[0043] The solubility of table 1 andrographolide in different solvents
[0044]
[0045]
[0046] From the above results, it can be seen that absolute ethanol has a low boiling point and has volatil...
Embodiment 10-15
[0048] Different proportions of F127 solidified andrographolide solution
[0049] prescription
[0050] Andrographolide 0.1g
[0051] NMP 400 μL
[0052] F127 0.1~0.6g
[0053] Weigh 0.1 g of andrographolide, add 400 μL of NMP to dissolve it ultrasonically, then add different masses of F127, heat and stir at 65°C until it melts, stop heating and continue stirring until it cools and solidifies to obtain a solidified solution of andrographolide containing F127.
[0054] Table 3 Adding different proportions of F127 solidified andrographolide solution at room temperature
[0055] F127 added amount State of the obtained andrographolide solidified solution at room temperature Example 10 0.1g It is in a liquid state at room temperature, and does not meet the requirement of being a solid solution at room temperature. Example 11 0.2g It is in a liquid state at room temperature, and does not meet the requirement of being a solid solution at room temperat...
Embodiment 16-21
[0057] Different proportions of Compritol 888ATO solidified andrographolide solution
[0058] Prescription Andrographolide 0.1g
[0059] NMP 400 μL
[0060] Compritol 888ATO 0.1~0.6g
[0061] Weigh 0.1 g of andrographolide, add 400 μL of NMP to dissolve it ultrasonically, then add different masses of Compritol 888ATO, heat and stir at 65°C until it melts, stop heating and continue stirring until it cools and solidifies to obtain a solidified andrographolide solution containing Compritol 888ATO.
[0062] Table 4 Add different proportions of Compritol 888ATO to solidify andrographolide solution at room temperature
[0063]
[0064] Table 5 solidified andrographolide solution in vitro release results under non-sink conditions
[0065]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com