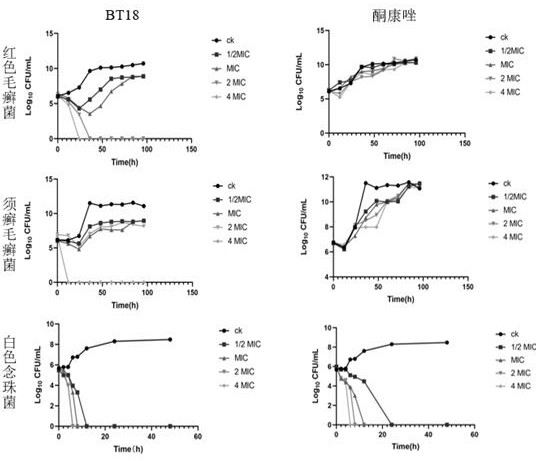
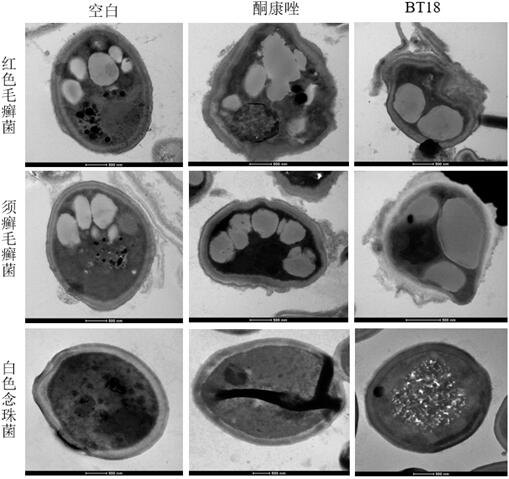
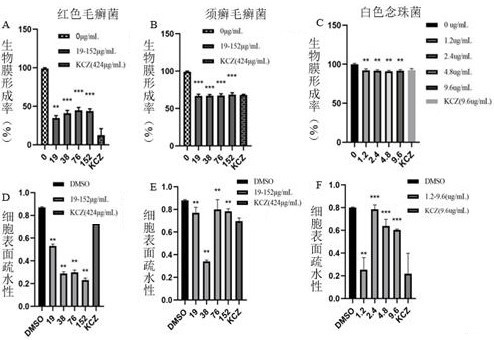
Application of evodiamine derivative in preparation of medicine for treating superficial fungal infection
A technology for evodial and fungal infection, which is applied in the field of medicine and can solve the problems of narrow antibacterial spectrum, inability to meet the needs of patients for medication, and large toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 14-ethyl-8,13,13b,14-tetrahydroindolo[2',3':3,4]pyrido[2,1-b]quinazolin-5(7H)-one (compound BT1)
[0029] The structural formula of compound BT1 is:
[0030] (1) At room temperature, dissolve methyl 2-(ethylamino)benzoate (compound 1-1, 1 mmol), tryptamine (1.5 mmol), and trimethylaluminum (3.0 mmol) in 10 ml of dichloromethane, in 0 ℃ for 6 hours, the product N-(2-(1 H -indol-3-yl)ethyl)-2-(ethylamino)benzamide (compound 1-2), the yield was 71%.
[0031] The structural formula of compound 1-1 is:
[0032] The structural formula of compound 1-2 is:
[0033] (2) N-(2-(1H-indol-3-yl)ethyl)-2-(ethylamino)benzamide (compound 1-2, 1 mmol), triethyl orthoformate (3 mmol) and boron trifluoride ether (0.5 mmol) was added to a 25 mL round bottom flask, and N,N-dimethylformamide was used as the solvent, replaced by argon, and reacted for 6 hours. After the completion of the reaction monitored by TLC, the N,N-dimethylformamide was extracted and washed with water and ethyl...
Embodiment 2
[0035] 14-isobutyl-8,13,13b,14-tetrahydroindolo[2',3':3,4]pyrido[2,1-b]quinazolin-5(7H)-one (Compound BT2)
[0036] The structural formula of compound BT2 is:
[0037] The raw material methyl 2-(ethylamino)benzoate (compound 1-1) in step (1) of Example 1 was replaced with methyl 2-(isobutylamino)benzoate (compound 2-1), and the remaining steps were prepared in the same way as in Example 1. Yield 73%. 1 H NMR (400 MHz, Chloroform- d ) δ 8.20 (s, 1H), 8.06 (dd, J = 7.8, 1.6 Hz,1H), 7.55 (d, J = 7.8 Hz, 1H), 7.44 – 7.36 (m, 2H), 7.25 – 7.20 (m, 1H), 7.15(t, J = 7.5 Hz, 1H), 7.11 – 7.02 (m, 2H), 5.92 (s, 1H), 4.86 (m, J = 13.1,5.6, 1.9 Hz, 1H), 3.30 (m, 1H), 3.08 (m, 1H), 3.00 – 2.82 (m, 3H), 2.71 (dd, J = 13.5, 9.5 Hz, 1H), 0.99 (d, J = 6.5 Hz, 3H), 0.77 (d, J = 6.7 Hz, 3H). 13 CNMR (101 MHz, CDCl 3 ) Δ 165.27, 148.73, 136.28, 132.72, 129.59, 129.07,126.57, 122.75, 122.65, 122.34, 120.59, 119.84, 118.72, 111.30, 70.54, 58.18, 27.23, 19.8.8, 19.8, 19.8, 19.8, 19...
Embodiment 3
[0040] 14-allyl-8,13,13b,14-tetrahydroindolo[2',3':3,4]pyrido[2,1-b]quinazolin-5(7H)-one (compound BT3)
[0041] The structural formula of compound BT3 is:
[0042] The raw material methyl 2-(ethylamino)benzoate (compound 1-1) in step (1) of Example 1 was replaced with methyl 2-(allylamino)benzoate (compound 3-1), and the remaining steps were prepared in the same way as in Example 1. The yield is 80%. 1 H NMR (400 MHz, Chloroform- d ) δ 8.56 (s, 1H), 8.11 (dd, J = 7.8, 1.6 Hz,1H), 7.59 (dd, J = 7.8, 1.1 Hz, 1H), 7.47 – 7.38 (m, 2H), 7.28 – 7.24 (m,1H), 7.20 – 7.05 (m, 3H), 5.99 (t, J = 1.6 Hz, 1H), 5.82 (m, J = 16.9, 10.2,6.6, 5.3 Hz, 1H), 5.04 (m, J = 10.2, 1.4 Hz, 1H), 4.94 (m, J = 17.0, 1.5 Hz,1H), 4.87 (m, J = 12.9, 4.9, 2.3 Hz, 1H), 3.69 (m, J = 15.8, 5.4, 1.7 Hz,1H), 3.50 (m, J = 15.8, 6.6, 1.2 Hz, 1H), 3.26 (m, J = 12.9, 10.5, 5.2 Hz,1H), 3.05 – 2.89 (m, 2H). 13 C NMR (101 MHz, CDCl 3 ) Δ 165.06, 148.39, 136.44,134.84, 132.77, 128.84, 128.11, 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com