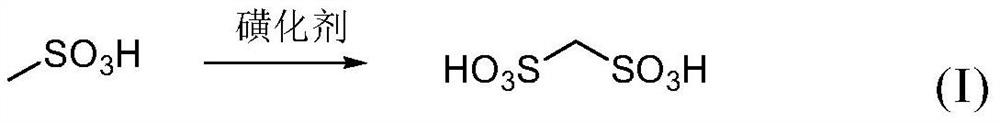Methylene methanedisulfonate and preparation method thereof
A technology of methylene disulfonate and methane disulfonic acid, applied in organic chemistry, etc., can solve the problems of low purity of methylene disulfonate, lower reaction yield, difficulty in solvent recovery, etc., and achieve easy continuous production Effects of production, improvement of reaction efficiency, and shortening of reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The invention provides a kind of preparation method of methylene disulfonate, which comprises the following steps:
[0045] (1) sulfonation reaction steps
[0046] performing a sulfonation reaction with methanesulfonic acid and a sulfonating reagent to obtain a sulfonation reaction solution containing methanedisulfonic acid;
[0047] The reaction equation involved in the sulfonation reaction is shown in formula (I).
[0048]
[0049] (2) Ring-closing reaction steps
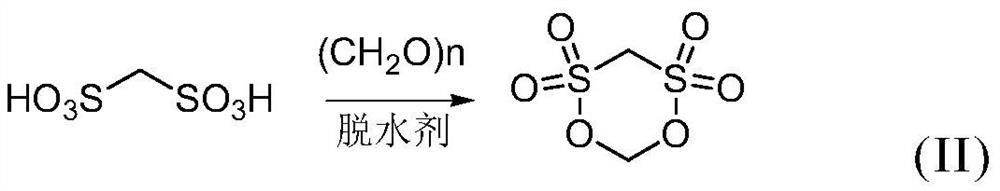
[0050] The sulfonation reaction solution obtained in step (1) and the condensation reagent are subjected to a ring-closing reaction under the action of a dehydrating agent sulfur trioxide, and cooled to obtain a crude methylene disulfonate;
[0051] The reaction equation involved in the ring-closing reaction is shown in formula (II).
[0052]
[0053] (3) Purification step
[0054] Purify the methylene disulfonate crude product obtained in step (2) to obtain the methylene disulfonate product.
[00...
Embodiment 1
[0091] (1) Sulfonation reaction
[0092] At 30° C., 9.61 g (0.100 mol) of methanesulfonic acid was added into a 200 mL four-necked pressure bottle, replaced with nitrogen three times to remove the air in the reaction system, and replaced with a nitrogen atmosphere. Stir at 100rpm, slowly add 8.0g (0.100mol) of sulfur trioxide liquid dropwise, exothermic during the dropwise addition, control the system temperature by cooling to 30°C, seal the four-necked pressure-resistant bottle after the dropwise addition, and heat up to 60°C, react for 24h. After the reaction was completed, it was cooled to 30° C. to obtain a white solid containing methanedisulfonic acid, which was detected by an ion chromatograph (Wantong 930) to confirm that the reaction of the methanesulfonic acid raw material was complete.
[0093] (2) Ring closing reaction
[0094] To the white solid containing methanedisulfonic acid obtained in step (1), slowly add 8.0 g (0.100 mol) of sulfur trioxide liquid dropwise...
Embodiment 2
[0098] (1) Sulfonation reaction
[0099] At 30° C., 9.61 g (0.100 mol) of methanesulfonic acid was added into a 200 mL four-necked pressure bottle, replaced with nitrogen three times to remove the air in the reaction system, and replaced with a nitrogen atmosphere. Stir at 100rpm, slowly add 40.0g (0.500mol) of sulfur trioxide liquid dropwise, release heat during the dropwise addition, control the system temperature by cooling to 30°C, seal the four-necked pressure-resistant bottle after the dropwise addition, and heat up to 160°C, react for 1h. After the reaction is completed, cool to 30°C to obtain a sulfonation reaction solution containing methanedisulfonic acid. The system is a brown liquid. It is detected by ion chromatography (Wantong 930) to confirm that the reaction of the methanesulfonic acid raw material is complete.
[0100] (2) Ring closing reaction
[0101] Under stirring, to the sulfonation reaction liquid obtained in step (1), control the temperature at 30°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com