Echinococcus granulosus recombinant protein and preparation method thereof
A technology of Echinococcus granulosus and recombinant protein, applied in the field of genetic engineering, can solve the problems of affecting protein conformation, inducing immune pathological effects, toxic and side effects, etc., achieving the effects of simple genetic manipulation, rapid cell reproduction and low culture cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Modification method of Echinococcus granulosus immunogenic protein EG95
[0042] On the basis of the amino acid sequence of the complete EG95 protein, the signal peptide sequence of 16 amino acids was removed from the N-terminal, and the transmembrane hydrophobic domain of 18 amino acids was truncated from the C-terminal, and the codon usage was used according to the prokaryotic expression system of E. coli Codon optimization was performed on the full-length gene of EG95 preferentially to improve protein expression and enhance its hydrophilicity. The modified EG95 protein is a recombinant protein of Echinococcus granulosus, named EG95s, and the nucleotide sequence (5'-3') of the gene encoding EG95s is as follows:
[0043] CAAGAATATAAAGGTATGGGTGTTGAAACCCGTACCACCGAAACACCGCTGCGTAAACATTTTAATCTGACACCGGTTGGTAGCCAGGGTATTCGTCTGAGCTGGGAAGTTCAGCATCTGAGCGATCTGAAAGGCACCGATATTAGCCTGAAAGCAGTTAATCCGAGCGATCCGCTGGTGTATAAACGTCAGACCGCAAAATTTAGTGATGGCCAGCTGACCATTGGTGAACTGAAACCGA...
Embodiment 2
[0047] Embodiment 2: the method for preparing EG95s recombinant protein
[0048] Step 1. Construct the modified EG95s gene sequence according to the method in Example 1.
[0049] Step 2, construction of recombinant expression plasmid pET28a-EG95s
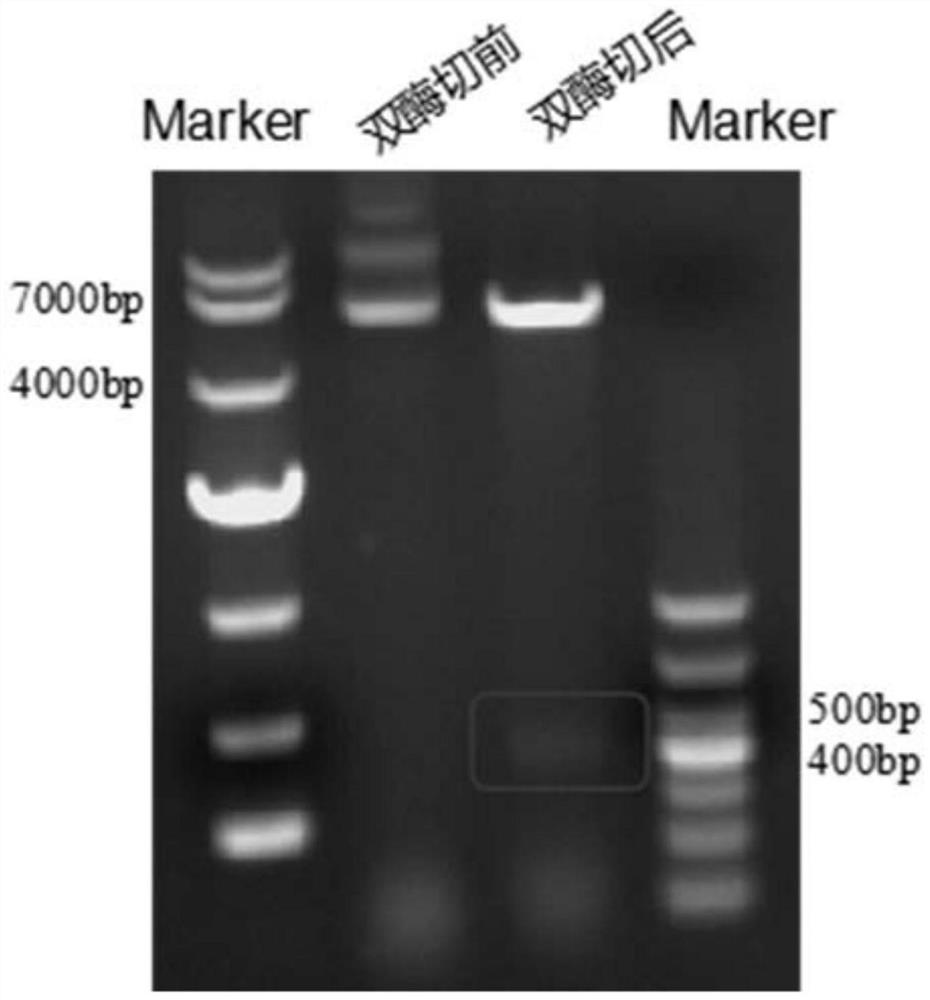
[0050] ① The modified EG95s gene sequence was gene-synthesized, and after PCR amplification, it was connected to the pET-28a vector (TaKaRa, Japan) through BamHI and XhoI (TaKaRa, Japan) restriction sites to construct the pET28a-EG95s expression vector. During the above PCR amplification, the primer pairs used are:
[0051] Upstream primer: 5'-GGATCCCAAGAATATAAAGGTATGGGT-3' (SEQ ID NO: 3);
[0052] Downstream primer: 5'-CTCGAGTGCGCTACCGCT-3' (SEQ ID NO: 4).
[0053] ②Take Escherichia coli BL21(DE3) competent cells (Qingke Biotech, China) out of the -80°C refrigerator and place them on ice for 2 minutes. When the competent cells melt, add 2uL pET28a-EG95s expression vector and mix gently Ice-bathed for 30 minutes, then placed in ...
experiment example 1
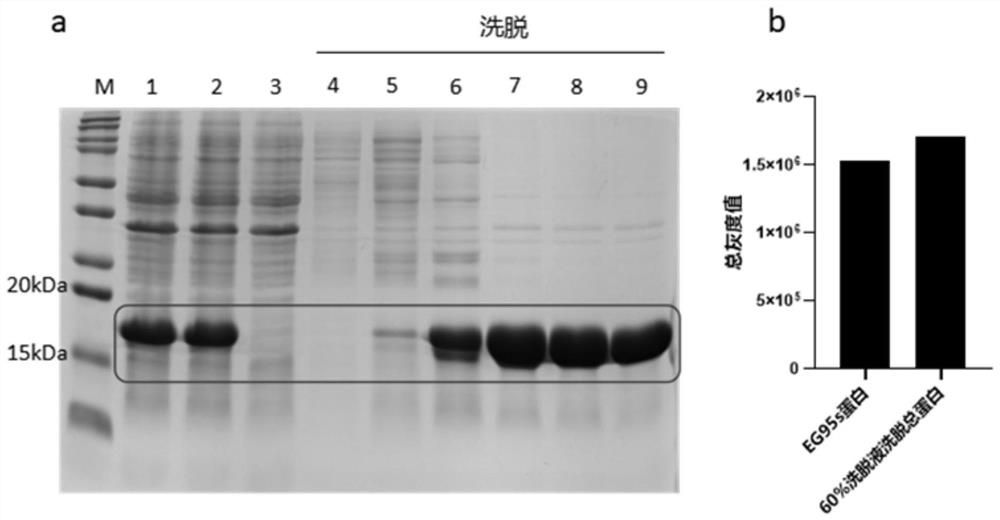
[0061] Experimental example 1: SDS-PAGE analysis of EG95s recombinant protein
[0062] 1. Experimental method
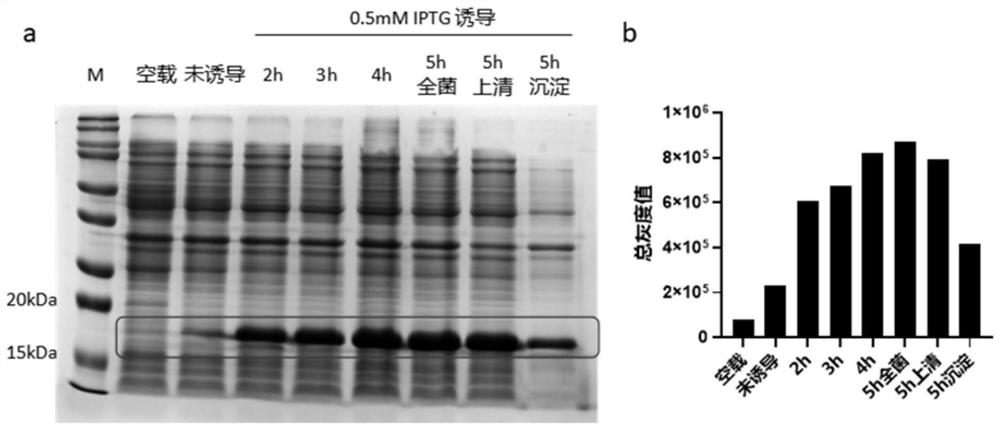
[0063] (1) Expression of EG95s
[0064] Referring to the method in step 3 of Example 2, the recombinant expression strain EG95s-1 was inoculated into LB culture medium containing 20 μg / mL kana, and cultivated to the OD of the bacterial solution 600 At 6 o'clock, IPTG was added to a final concentration of 0.5 mM, and then placed on a shaker at 37° C. at 200 rpm to induce expression, and the bacterial liquid was collected every 1 h. Centrifuge the collected bacterial solution for 20 minutes under the condition of centrifugal force of 13000g, collect the precipitate and resuspend it with 20mM PBS, ultrasonically lyse it on ice (power 300w, work 5s, interval 5s, total time 20min), add 5x to 1 / 4 volume Protein loading buffer (NCM Biotech, China), boiled in a metal bath at 100°C for 10 minutes, centrifuged at 12,000 g for 5 minutes, and the whole bacteria, supernatant, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com