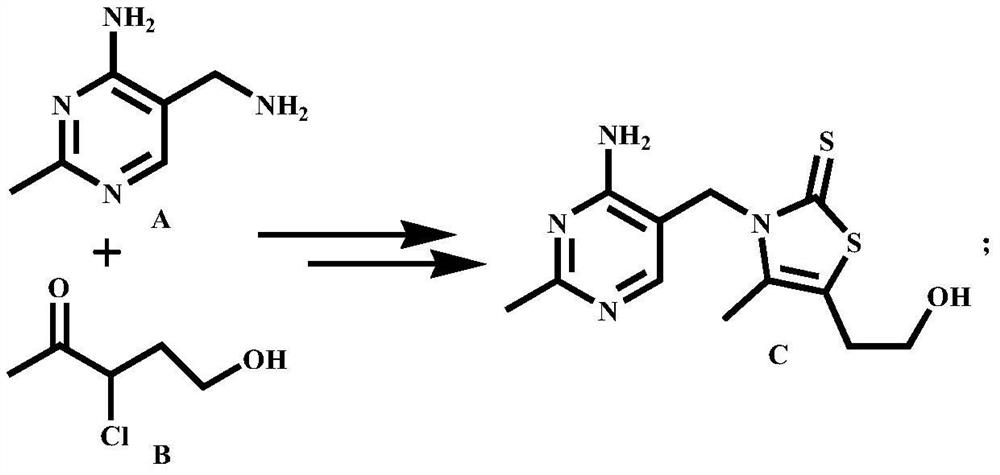
Green synthesis method of vitamin B1 intermediate
A synthesis method and compound technology, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve problems such as difficulty in controlling the amount of chlorinated reagents, troublesome post-processing, environmental hazards, etc., and achieve industrialization. The effect of application, less corrosiveness of equipment and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
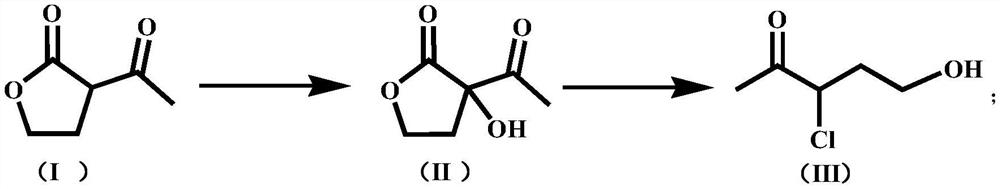
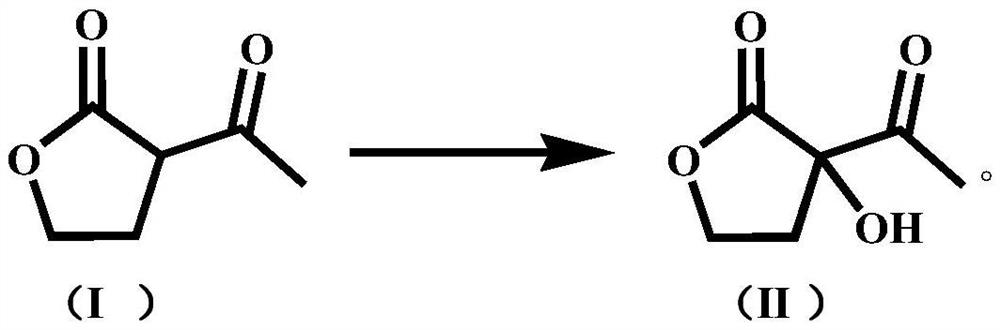
[0053] Take 220g H 2 O, 40g α-acetyl-γ-butyrolactone (0.3122mol), 0.618g cuprous chloride, 0.786g manganese chloride, add in a 500mL tetrafluoroethylene-lined airtight autoclave with stirring and cooling reflux device , oxygen replacement 3 times, and then feed oxygen into the autoclave until the pressure in the autoclave is about 1.36MPa, and keep the pressure in the autoclave at about 1.36MPa, raise the temperature in the autoclave to 85°C, and react for 10h under stirring. After the reaction, the temperature in the kettle was slowly lowered to 25°C, and the oxygen in the kettle was evacuated, and then HCl was introduced until the initial reaction pressure in the kettle was 0.48MPa, then the introduction was stopped, and the reaction was continued for 2 hours under stirring. Then slowly heat up to 70°C, continue to react for 2h under stirring, after the reaction is over, cool to room temperature, connect the exhaust port of the autoclave to the tail gas absorption device and...
Embodiment 2
[0055] Take 220g H 2 O, 40g α-acetyl-γ-butyrolactone (0.3122mol), 0.618g cuprous chloride, 1.105g cobalt acetate, join in the 500mL tetrafluoroethylene-lined airtight autoclave with stirring and cooling reflux device, Oxygen was replaced 3 times, and then oxygen was introduced into the autoclave until the pressure inside the autoclave was about 1.36 MPa, and the pressure inside the autoclave was maintained at about 1.36 MPa. After the reaction, the temperature in the kettle was slowly lowered to 25°C, and the oxygen in the kettle was evacuated, and then HCl was introduced until the initial reaction pressure in the kettle was 0.32 MPa, then the introduction was stopped, and the reaction was continued for 2 hours under stirring. Then slowly heat up to 70°C, continue to react for 2h under stirring, after the reaction is over, cool to room temperature, connect the exhaust port of the autoclave to the tail gas absorption device and open it, take a sample after emptying, and use the...
Embodiment 3
[0057] Take 300g H 2 O, 40g α-acetyl-γ-butyrolactone (0.3122mol), 0.618g cuprous chloride, 1.295g ruthenium trichloride, add to a 500mL closed autoclave lined with tetrafluoroethylene with stirring and cooling reflux device In the autoclave, oxygen was replaced three times, and then oxygen was introduced into the autoclave until the pressure in the autoclave was 0.85MPa, and the pressure in the autoclave was maintained at about 0.85MPa. After the reaction, the temperature in the kettle was slowly lowered to 25°C, and the oxygen in the kettle was evacuated, and then HCl was introduced until the initial reaction pressure in the kettle was 0.32 MPa, then the introduction was stopped, and the reaction was continued for 2 hours under stirring. Then slowly heat up to 70°C, continue to react for 2h under stirring, after the reaction is over, cool to room temperature, connect the exhaust port of the autoclave to the tail gas absorption device and open it, take a sample after emptying,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com