Thermomyces lanuginosus lipase mutant G91C and application thereof
A thermophilic fungus and mutant technology, applied in the field of enzyme engineering, can solve the problems of difficult purification, high price, limited industrial production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The mutation site of TLL lipase was predicted by molecular dynamics simulation and Discovery Studio 2017 software.
[0038]The 3D model of Thermomyces lanuginosus lipase (TLL) was obtained from the Protein Data Bank (PDB) (PDBID: 1DT3). The molecular dynamics simulation of TLL under the condition of water solvent was carried out by the molecular dynamics simulation software Maestro, so as to obtain the protein flexible region. Next, use Discovery Studio 2017 software to mutate all amino acids and calculate the mutation energy to predict the thermostable mutation site. According to the prediction analysis, finally obtained 6 mutants, namely G246C, G246R, G91C, G91I, G91K and G91T, wherein the gene sequence of G246C is shown in SEQ ID NO.1, and the gene sequence of G246R is shown in SEQ ID NO.2. The gene sequence of G91C is shown in SEQ ID NO.3, the gene sequence of G91I is shown in SEQ ID NO.4, the gene sequence of G91K is shown in SEQ ID NO.5, and the gene sequence of ...
Embodiment 2
[0040] Using the pPICZαA-TLL recombinant plasmid as template DNA, PCR primers were designed according to the TLL-protll nucleotide sequence (as shown in SEQ ID NO.8). The mutants are mutated into target amino acids at different structural positions, and the mutations at the amino acid positions need to be synthesized using Pichia pastoris-biased codons. The plasmid used in the present invention is the pPICZαA plasmid, which is the latest secreted expression plasmid of Pichia pastoris. Its signal peptide comes from Saccharomyces cerevisiae α-factor, which can guide the secretion of recombinant protein to the extracellular space, and often shows protein expression in the actual production process. Compared with higher characteristics. As a plasmid with a marker gene, which includes a Zeocin resistance marker gene, it can be used as a marker for transformant screening, and can provide help and convenience for transformant screening during actual operation. The gene synthesis par...
Embodiment 3
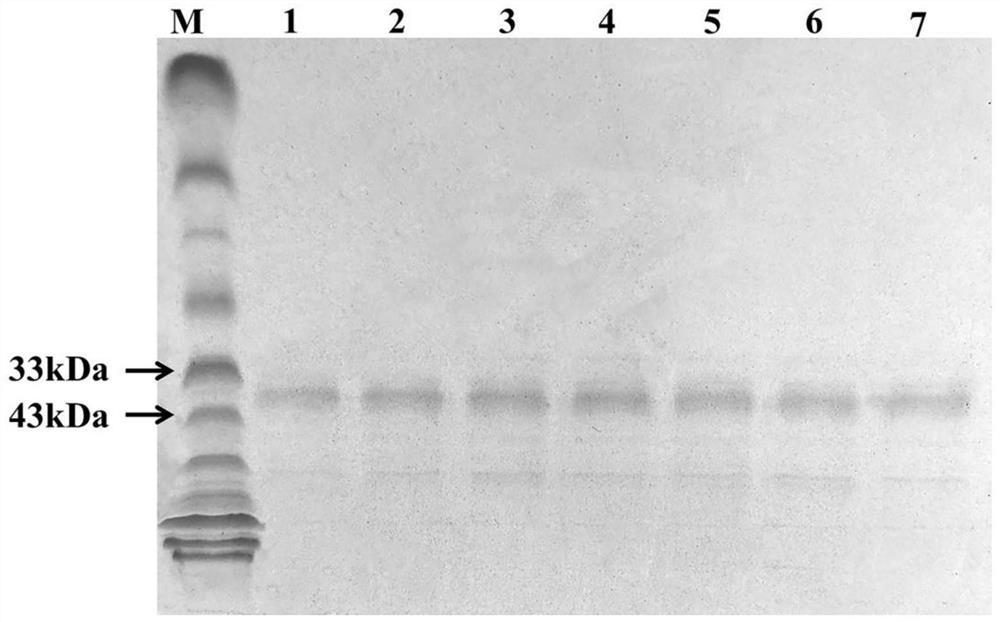
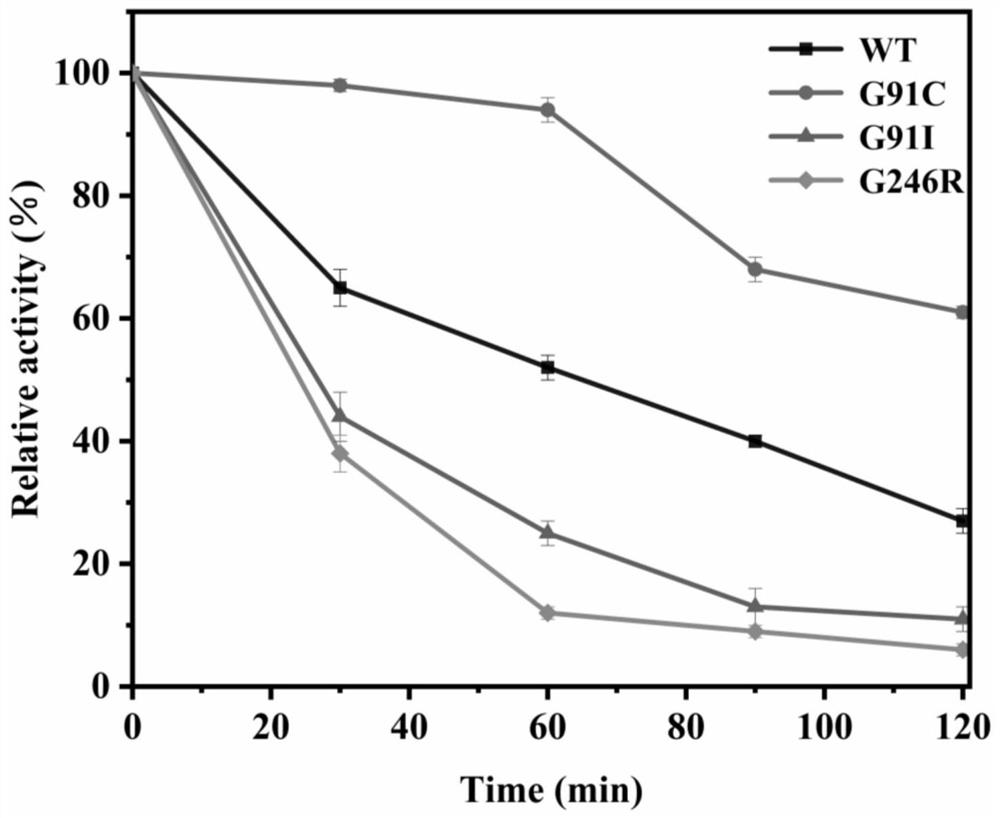
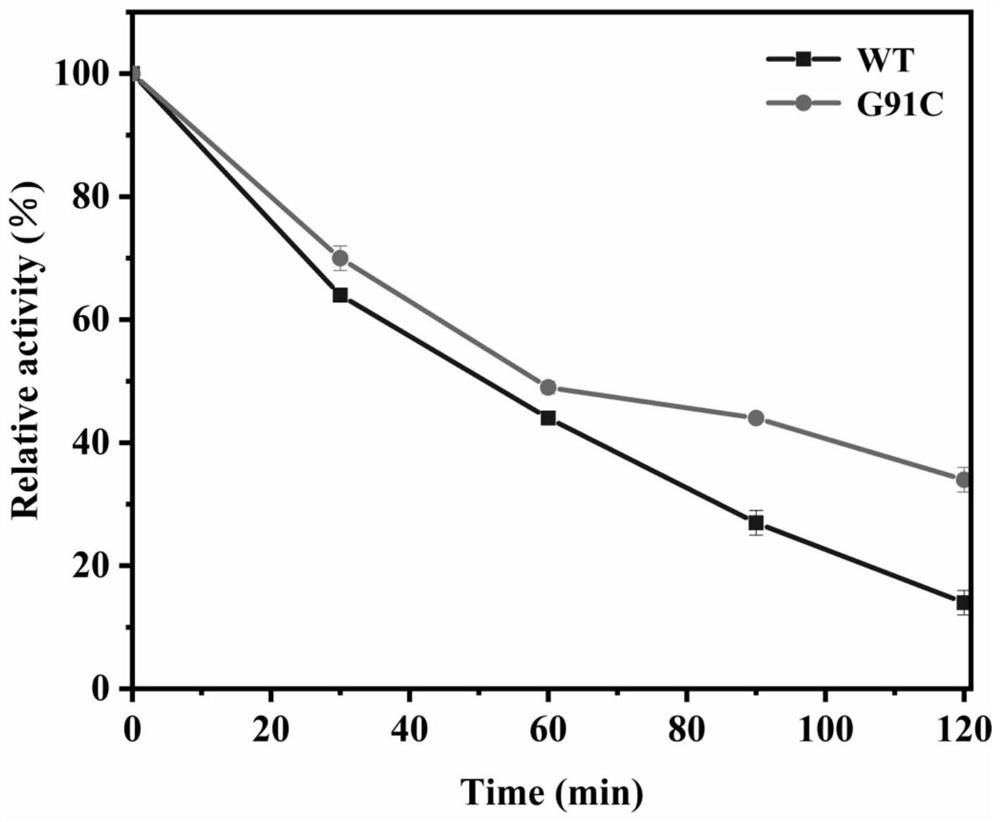
[0044] The definition of one enzyme activity unit: under the reaction conditions of pH = 7.5 and 40°C, the amount of enzyme required to hydrolyze the substrate p-nitrophenyl laurate (C12) to generate 1 μmoL of p-nitrophenol per minute is one enzyme activity The unit is represented by U. Aspirate 180 μL of PBS (pH=7.5) as the buffer solution, and then add 10 μL of 20 mmol / L substrate p-nitrophenyl laurate (C12), mix thoroughly and incubate in a water bath at 40°C for 5 minutes. Then add 10 μL to dilute to a suitable multiple for measuring enzyme activity, accurately reflect for 5 minutes, and finally add 600 μL of Na 2 CO 3 solution to terminate the reaction. Centrifuge at 12,000 rpm for 3 minutes, and add 200 μL of reaction solution to each microtiter plate. Finally, the absorbance was measured at 410 nm with a microplate reader. The enzyme activities of wild-type TLL and 6 mutants are shown in Table 1 and .
[0045] Table 1 Enzyme activity comparison between wild-type TL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com