Application of hypocrellin B to preparation of medicine for preventing and treating African swine fever
A technology of Hypocretin B and African swine fever, applied in the field of biomedicine, can solve the problems of unreported and unseen effects of African swine fever virus, and achieve the effects of mature and reliable production technology, clear mechanism of action, and easy transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
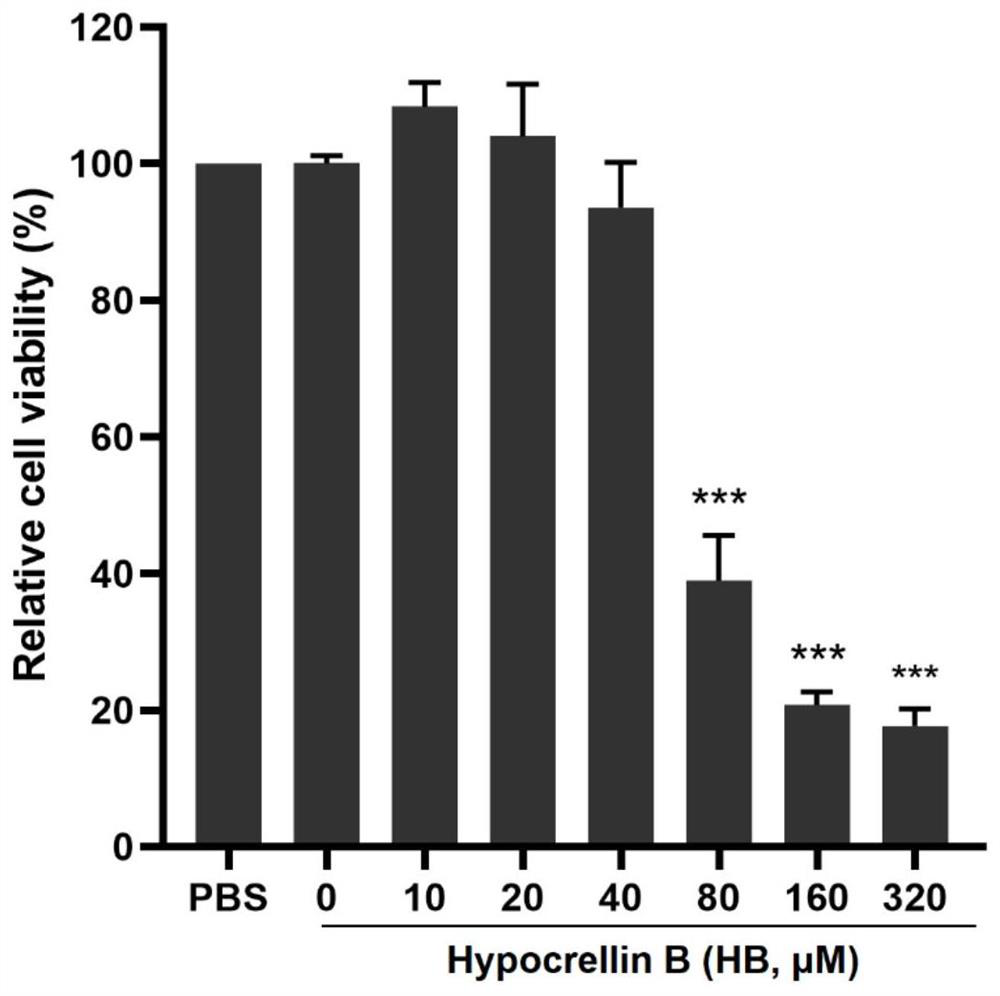
[0035] Example 1 Hypocretin B Cytotoxicity Test on PAM Cells
[0036] 1. Experimental method
[0037] Dilute PAM cells at 1 x 10 6 Cells / mL were cultured in 96-well cell culture plates containing RPMI-1640 medium (containing penicillin 100 U / mL, streptomycin 50 μg / mL, amphotericin 0.25 μg / mL) containing 10% FBS. 37°C, 5% CO 2 Incubate for 6 h in the incubator. When it adheres to the wall by 70% to 80%, discard the culture medium, wash twice with PBS (phosphate buffered saline), and dilute hypocrellin B (0, 20 , 40, 80, 160 and 320 μM), 100 μL per well, set blank cell group, set 6 parallel wells for each compound concentration, 37 ° C, 5% CO 2 After culturing in the incubator for 48 hours, the culture medium was discarded, washed twice with PBS, new culture medium was added to each well, and 10 μL of CCK8 reagent was added to each well, and after 1 hour of dark incubation, the absorbance of the cells was detected at a wavelength of 450 nm.
[0038] 2. Experimental results ...
Embodiment 2
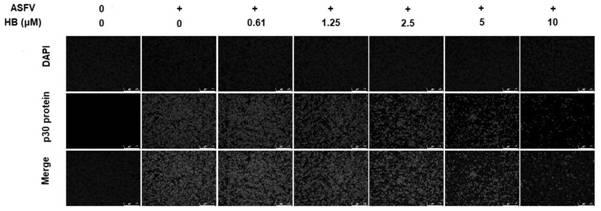
[0040] Example 2 Effect of Hypocretin B Treatment of Different Concentrations on the Expression of ASFV p30 Protein in PAM Cells
[0041] 1. Experimental method
[0042] Inoculate PAM cells in a 96-well cell culture plate, 100 μL per well, at 37 °C, 5% CO 2 Culture in an incubator until 70% to 80% adhere to the wall. Discard the culture medium, inoculate 0.1MIO of ASFV virus solution, 100 μL per well, set a blank control group at the same time, 37 ° C, 5% CO 2 After incubation in the incubator for 2 h, the supernatant was discarded, and culture solutions containing different concentrations (2.5 μM, 5 μM, 10 μM) of hypocrellin B were added to continue culturing. After 48 hours, discard the supernatant, add 100 μL of 4% paraformaldehyde to each well, and fix at room temperature for 15 minutes; discard the fixative solution, wash with PBS three times, each time for 5 minutes; add 100 μL of 0.3% Trition-X100 solution to each well, and incubate at room temperature 10 min; wash t...
Embodiment 3
[0046] Example 3 Effect of Hypocretin B on the Viral Titer of ASFV
[0047] 1. Experimental method
[0048] 1. Treatment of ASFV-infected PAM cells with hypocrellin B
[0049] PAM cells were seeded in 24-well cell culture plates at 37°C, 5% CO 2 Culture in an incubator until 70% to 80% adhere to the wall, and discard the culture medium. Insert 500 μL of ASFV (MIO=0.1) into each well, and set a blank control group at the same time, after incubating at 37°C for 2 hours, wash twice with PBS, add 500 μL of culture solution containing different concentrations of hypocrellin to each well, and set the virus at the same time Control group, placed at 37°C, 5% CO 2 After 48 h in the incubator, the culture was terminated. Cell samples were collected after repeated freezing and thawing at -80°C and 4°C three times.
[0050] 2. The virus titer (HAD 50 ) determination
[0051] PAM cells infected with ASFV treated with hypocretin B were inoculated in a 96-well plate, 100 μL per well, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com