Synthesis method of ethyl tetrahydrofurfuryl ether
A technology of tetrahydrofurfuryl alcohol and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of insecurity, low ETFE separation efficiency, low dehydration efficiency, etc., and achieve the effects of easy production control, easy process control, and high dehydration efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
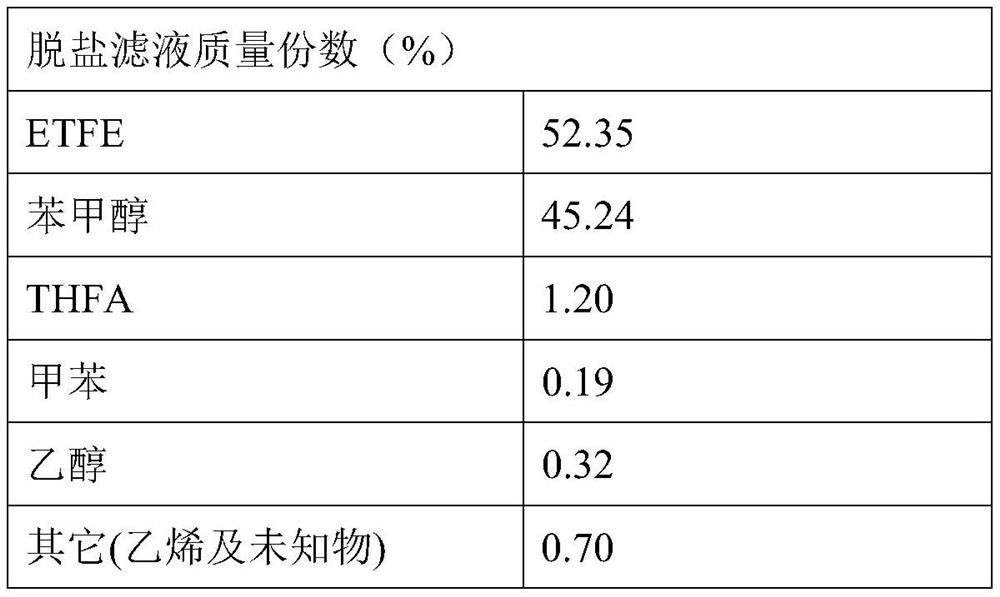
[0047] Add 1028g of THFA, 450g of caustic soda of 99.0%, 1200mL of benzyl alcohol of 98.6% and 99 % of 40mL toluene, stirred and heated to 100-125°C for dehydration reaction, when no obvious water escaped from the azeotropic toluene in the water separator, the dehydration reaction time was 8.5h.
[0048] Then the dehydrated reactant is pressed into a condensation (or coupling) reactor with a cooling system, and the material is lowered to 0-10°C with -5°C brine, and the alkoxide presents a yellow viscous resinous fluid. Add 755g of 99.3% ethyl chloride into the condensation reaction kettle at a constant speed within 2 hours to carry out the condensation reaction, and at the same time remove the heat of reaction with brine, control the condensation reaction temperature not higher than 20°C, and the reaction pressure not higher than 0.2MPa, After the addition of ethyl chloride, the reaction was continued for another 2 hours. At this time, the pressure in the kettle dropped to zer...
Embodiment 2
[0054] The relevant process conditions and operating methods in Example 1 were kept unchanged, and only relevant parameters were adjusted in each unit.
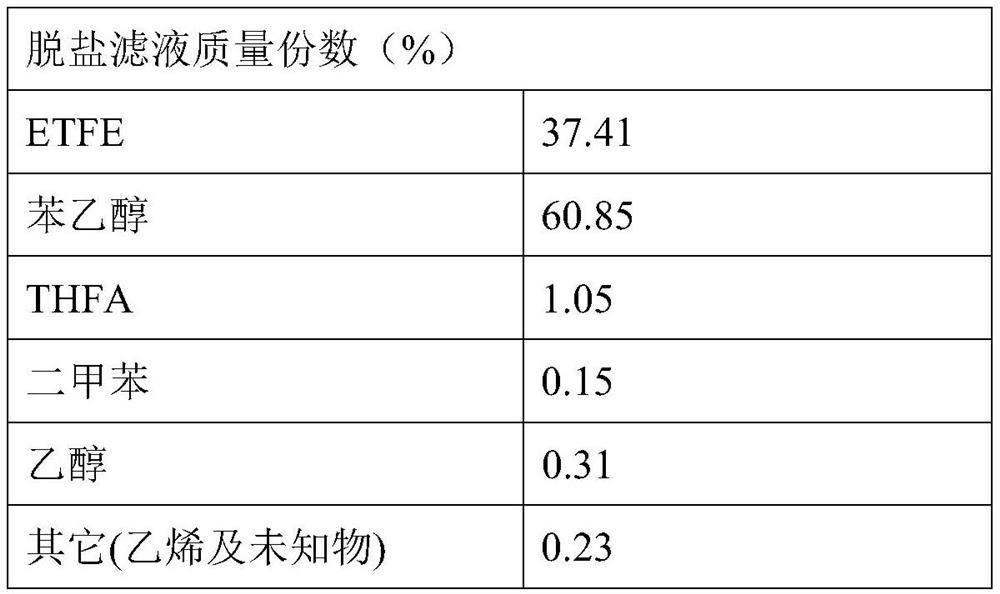
[0055] For example, in the dehydration reaction unit, it is set to add 2000mL of phenylethyl alcohol with a mass fraction of 98.2%, 50mL of xylene, and 460g of caustic soda, and the dehydration reaction temperature is 120-135°C. flowing liquid;
[0056] For example, in the condensation reaction unit, 1253 g of ethyl bromide with a mass content of 99.6% is used instead, and the reaction pressure is not higher than 0.1 MPa.
[0057] Results After filtering the salt-containing crude product at room temperature to remove sodium chloride, 3243 g of the filtrate of the salt-free crude product was obtained. The yield of ETFE was 95.15%.
[0058] Table 2
[0059]
[0060] Put the filtrate in a three-necked flask equipped with a silver-plated glass rectification column with high-efficiency packing about 45 plates for rectificati...
Embodiment 3
[0062] The relevant process conditions and operating methods in Example 1 remained unchanged, and only relevant adjustments were made in each unit.
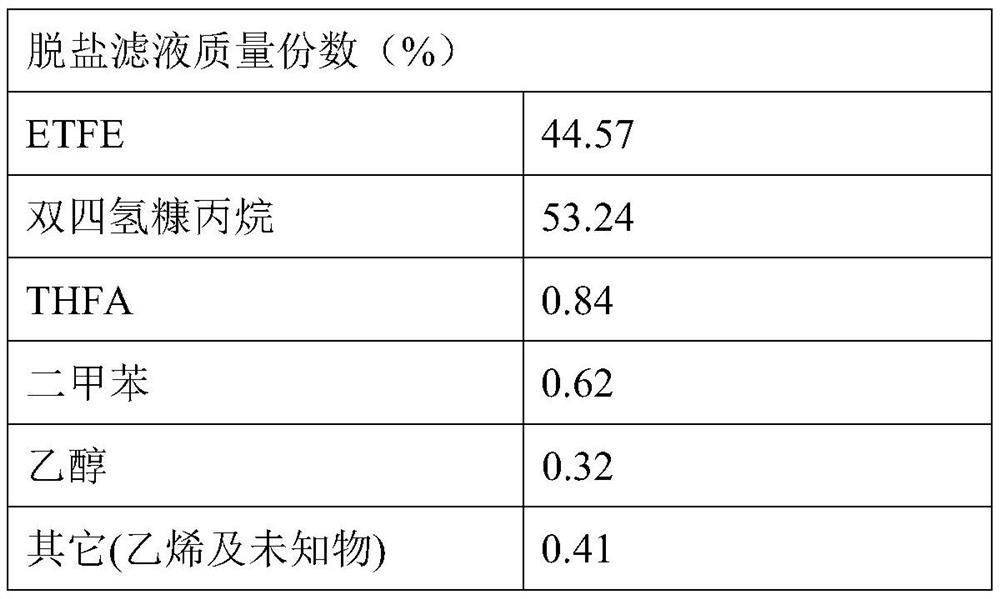
[0063] For example, in the dehydration reaction unit, it is set to add 500g of caustic soda, 1500mL of ditetrahydrofurfurylpropane and 50mL of xylene, and the dehydration reaction temperature is 130-140°C. After a reaction time of 9 hours, the alkoxide presents a dark yellow and easy-flowing fluid;
[0064] For example, in the condensation reaction unit, the reaction temperature is 10-15°C, and the reaction pressure is not higher than 0.2MPa.
[0065] Results After the salt-containing crude product was filtered at room temperature to remove sodium chloride, 2714 g of the filtrate of the salt-free crude product was obtained. The yield of ETFE was 94.48%.
[0066] table 3
[0067]
[0068] The filtrate is placed in a three-necked flask equipped with a silver-plated glass rectification column with high-efficiency packing of abo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com