Method for detecting genetic toxic impurities of Afatinib dimaleate
A technology of afatinib maleate and genotoxicity, which is applied in the field of detection of genotoxic impurities of afatinib maleate, which can solve the problems of uncontrolled detection cost, low detection method sensitivity, and lack of in-depth research , to achieve the effects of short detection time, good peak shape symmetry, precision and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: chromatographic conditions and chromatographic system of detection method of the present invention
[0037] Instrument: Thermo Fisher High Performance Liquid Chromatography
[0038] Chromatographic column: YMC Triart C18 column (4.6mm×250mm, 3μm)
[0039] Mobile phase A: 0.01mol / L potassium dihydrogen phosphate solution, adjust the pH value to 3.0 with phosphoric acid
[0040] Mobile Phase B: Acetonitrile
[0041] The gradient program is as follows:
[0042] In terms of volume ratio, start elution with mobile phase A 75%, mobile phase B 25%, gradually reduce the proportion of mobile phase A, gradually increase the proportion of mobile phase B, until 25 minutes mobile phase A reaches 35%, mobile phase B reaches 65% %, continue elution for 10 minutes to 35 minutes, increase the proportion of mobile phase A, reduce the proportion of mobile phase B, and adjust mobile phase A to 75% and mobile phase B to 25% at 36 minutes, and continue elution for 9 minutes....
Embodiment 2
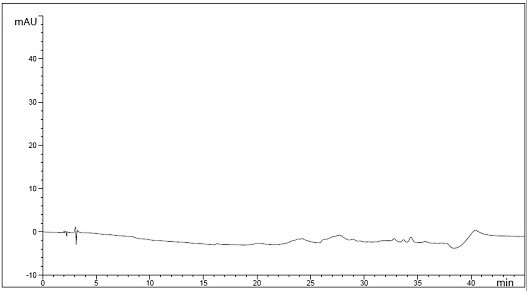
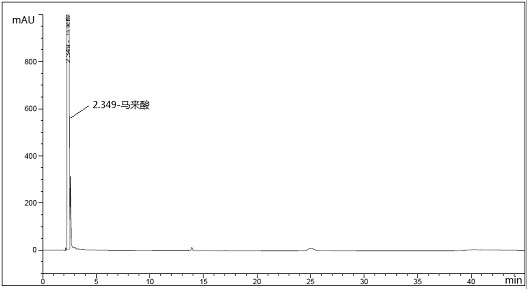
[0057] Embodiment 2: detection method specificity test of the present invention
[0058] Diluent: Acetonitrile-Water (30:70)
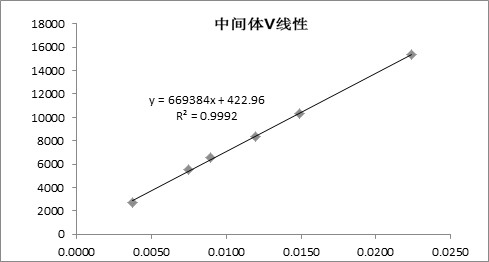
[0059] Intermediate V solution: Take an appropriate amount of Intermediate V, weigh it accurately, add a diluent to make a solution of about 5 μg per 1 mL.
[0060] Maleic acid positioning solution: Take an appropriate amount of maleic acid, weigh it accurately, add a diluent to make a solution containing about 200 μg per 1 mL, and use it as the maleic acid positioning solution.
[0061] Impurity Interference Solution: Take another appropriate amount of impurities A, B, C, D, E, F, G, H, and I, add diluent to make a mixed solution containing about 0.5 μg of each impurity per 1 mL as the impurity interference solution.
[0062] Afatinib maleate sample solution: take an appropriate amount of this product, weigh it accurately, add a diluent to make a solution containing about 0.5mg per 1mL, and use it as the afatinib maleate sample solution.
[0063] In...
Embodiment 3
[0067] Embodiment 3: Quantitation limit and detection limit test of detection method of the present invention
[0068] Limits of detection (LOD) and limits of quantitation (LOQ) were determined by the signal-to-noise ratio method. The intermediate V solution was diluted step by step, the measured signal was compared with the baseline noise, and the lowest concentration that could be reliably detected was calculated. The results are shown in Table 2.
[0069] Table 2: LOQ and LOD Results
[0070]
[0071] The quantitative limit concentration of intermediate V is lower than the quality control limit of intermediate V, and the detection limit concentration is lower than 1 / 10 of the quality control limit of intermediate V, ensuring that intermediate V can be effectively detected above the quality control limit of 1 / 10 out, from Table 2 and Image 6 It can be seen that the high performance liquid chromatography disclosed by the present invention can detect 2.6-8.0ppm of interm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com