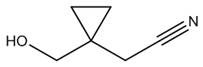
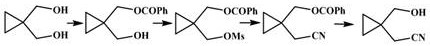
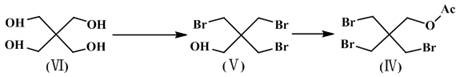
Preparation method of 1-hydroxymethyl cyclopropyl acetonitrile
A hydroxymethyl cyclopropyl acetonitrile and acetylation technology is applied in the field of preparation of 1-hydroxymethyl cyclopropyl acetonitrile, and can solve the problems of long distillation time, difficulty in rectifying and purifying the product, and increasing process cost. The effect of reducing the number of processing steps, shortening the reaction time, and reducing the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0040] In order to understand the technical content of the present invention more clearly, the following examples are given for detailed description, and the provided examples should not be construed as limiting the protection scope of the present invention. The raw material of compound VI pentaerythritol in the examples is a commercially available product.
[0041] Step 1: Preparation of tribromoneopentyl acetate (compound Ⅳ)
[0042] Add 200g of pentaerythritol (compound VI), 880g of hydrobromic acid (48%), 200g of concentrated sulfuric acid and 800g of acetic acid into a 3L three-neck flask, stir and raise the temperature (100-140°C) to react, and the reaction is completed in about 20 hours. The temperature was lowered to 50°C, and the layers were allowed to stand, and 100 g of acetic anhydride was added to the lower organic phase, and stirred and reacted at 60°C for 6 hours. Add 200g of water to the reaction solution, stir for half an hour, stand to separate the layers, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com