Synthesis method of aspoxicillin sodium
A technology of apocillin sodium and a synthetic method, applied in the field of apocillin sodium synthesis, can solve the problems of high production and synthesis cost, high price and the like, and achieve the effects of avoiding amino acid racemization reaction, simple method and avoiding environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
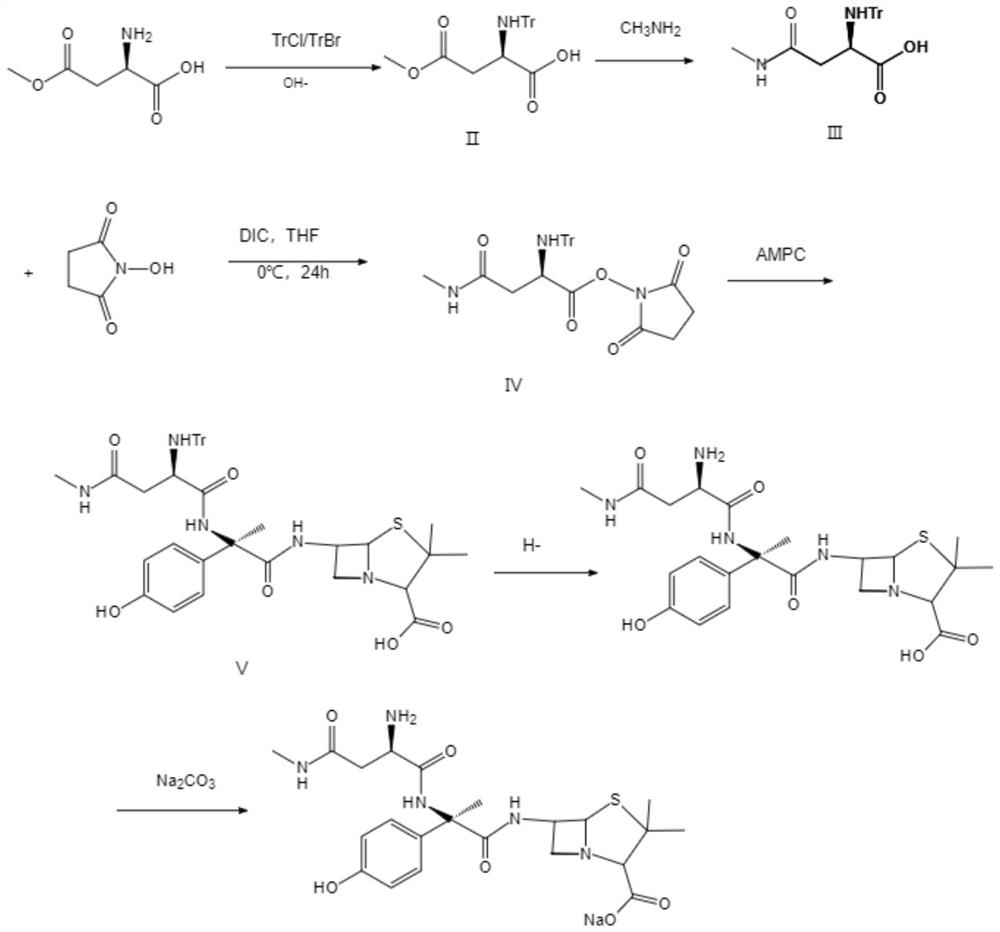
[0029] A kind of synthetic method of apoxicillin sodium, comprises the following steps:
[0030] (1) reacting D-aspartic acid-β-methyl ester with TrCl under alkaline conditions to obtain compound II;
[0031] (2) Compound II is methylated in an aqueous solution of methylamine to obtain Compound III;
[0032] (3) Compound Ⅲ and N-hydroxysuccinimide are reacted with tetrahydrofuran and N,N-diisopropylcarbodiimide to form compound Ⅳ;
[0033] (4) Prepared by reacting compound IV with amoxicillin triethylamine salt, compound V;
[0034] (5) Compound V removes the trityl group under mild acidic conditions to obtain apoxicillin;
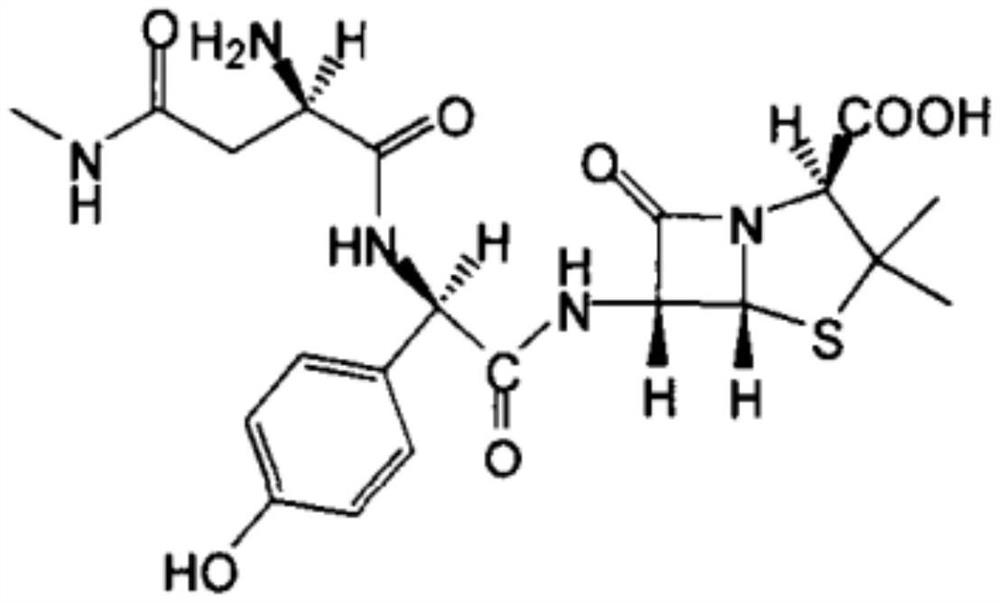
[0035] (6) Apoxicillin is reacted in a solution of N,N-dimethylformamide and sodium carbonate to obtain apoxicillin sodium.
[0036] Step (1) is specifically: D-aspartic acid-β-methyl ester is dissolved in the solution of methanol and sodium hydroxide, the pH is adjusted to 8 with hydrochloric acid, and TrCl is added dropwise while stirring at 0°C to mi...
Embodiment 2
[0043] A kind of synthetic method of apoxicillin sodium, comprises the following steps:
[0044] (1) reacting D-aspartic acid-β-methyl ester with TrCl under alkaline conditions to obtain compound II;
[0045] (2) Compound II is subjected to a methylation reaction in an aqueous solution of methylamine to obtain Compound III;
[0046] (3) Compound III and N-hydroxysuccinimide are reacted with tetrahydrofuran and N,N-diisopropylcarbodiimide to form compound IV;
[0047] (4) Prepared by reacting compound IV with amoxicillin triethylamine salt, compound V;
[0048] (5) Compound V removes the trityl group under mild acidic conditions to obtain apoxicillin;
[0049] (6) Apoxicillin is reacted in a solution of N,N-dimethylformamide and sodium carbonate to obtain apoxicillin sodium.
[0050] Step (1) is specifically: D-aspartic acid-β-methyl ester is dissolved in a solution of methanol and sodium hydroxide, the pH is adjusted to 9 with hydrochloric acid, and triphenylchloromethane i...
Embodiment 3
[0057] A kind of synthetic method of apoxicillin sodium, comprises the following steps:
[0058] (1) reacting D-aspartic acid-β-methyl ester with TrCl under alkaline conditions to obtain compound II;
[0059] (2) Compound II is subjected to a methylation reaction in an aqueous solution of methylamine to obtain Compound III;
[0060] (3) Compound III and N-hydroxysuccinimide are reacted with tetrahydrofuran and N,N-diisopropylcarbodiimide to form compound IV;
[0061] (4) Prepared by reacting compound IV with amoxicillin triethylamine salt, compound V;
[0062] (5) Compound V removes the trityl group under mild acidic conditions to obtain apoxicillin;
[0063] (6) Apoxicillin is reacted in a solution of N,N-dimethylformamide and sodium carbonate to obtain apoxicillin sodium.
[0064] Step (1) is specifically: D-aspartic acid-β-methyl ester is dissolved in a solution of methanol and sodium hydroxide, the pH is adjusted to 8 with hydrochloric acid, and triphenylchloromethane i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com