Tubeimoside with immunologic adjuvant effect as well as preparation method and application of tubeimoside
A technology of immune adjuvant, tubein C, is applied to tubei saponin with immune adjuvant function and the fields of preparation and use thereof, which can solve the problem of large toxic and side effects, affecting the nervous system of the body, lack of resources of Saponin in South America, etc. problems, to achieve the effects of no harm to the environment and the body, strong humoral immune response, and suitability for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Preparation of total saponins from Fritillaria japonicus
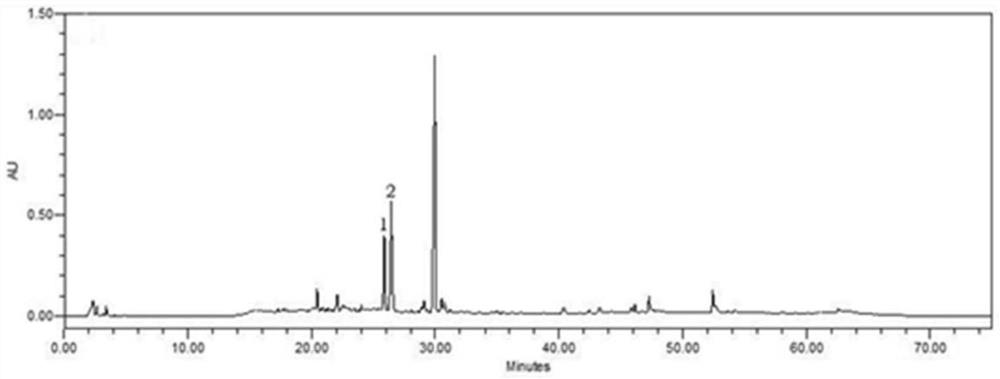
[0043] 1kg of the crude powder of the medicinal material Tuibei, reflux extraction with 70% ethanol for 3 times for 1.5 hours each time, filter, combine the filtrates, recover the ethanol under reduced pressure, and concentrate to obtain the alcohol extract. The alcohol extract was passed through a D101 macroporous adsorption resin column, and gradient elution was carried out with water and 10%-95% ethanol mixture. The eluent was checked by thin-layer chromatography, and the eluents with the same spots were combined, and the ethanol was recovered under reduced pressure, concentrated, and freeze-dried to obtain 26.24 g of off-white total glucosides. The HPLC chromatogram of the total saponins of Soil Fritillaria is shown in figure 1 .
Embodiment 2
[0044] Embodiment 2 Preparation of Tuimoside B
[0045] Weigh 1.0 g of total saponins from Fritillaria japonicus, add 5 mL of 50% ethanol to dissolve, filter through a 0.22 μm microporous membrane, and inject the filtrate into a preparative high-performance liquid chromatograph, the mobile phase is absolute ethanol-water (40:60), The flow rate was 10 mL / min, and the effluent from the chromatographic peak corresponding to Tuifumoside B was collected, the solvent was recovered under reduced pressure, and freeze-dried to obtain 98.9 mg of Tuifumoside B (I).
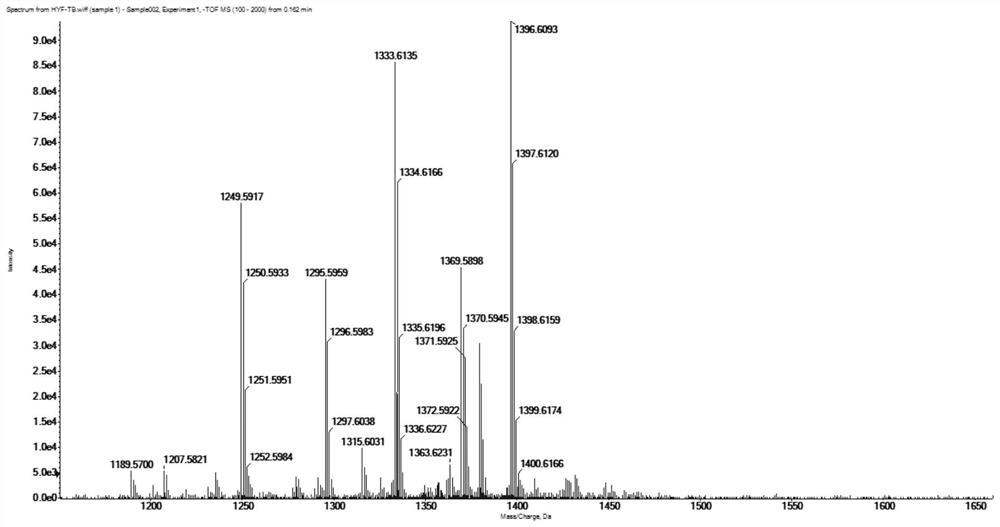
[0046] The molecular weight of the prepared tulimoside B was detected by an OrbitrapElite mass spectrometer (Thermo Scientific, Bremen, Germany). See figure 2 . The quasi-molecular ion peak of HR-ESI-MS m / z 1333.6135[M-H] - (calculated value: 1334.6143), molecular formula: C 63 h 98 o 30 .
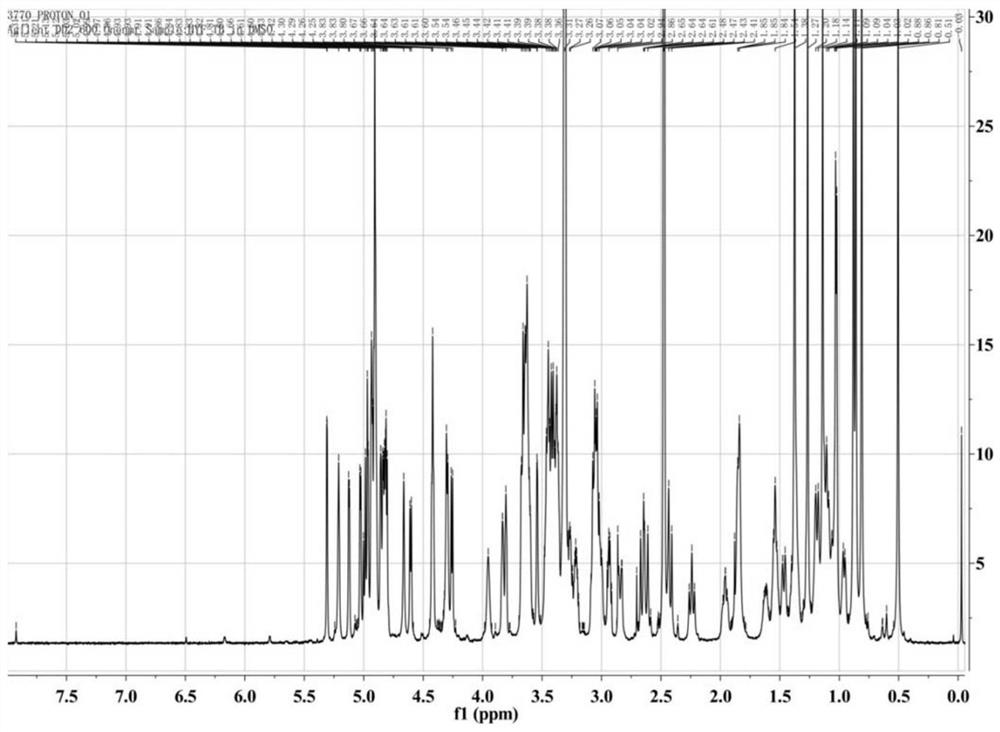
[0047] An Agilent DD2-600 nuclear magnetic resonance instrument (TMS as internal standard, DMSO-D 6 as a solvent) to detect th...
Embodiment 3
[0053] Example 3 Preparation of Tuimoside C
[0054] Weigh 1.0 g of total saponins from Fritillaria japonicus, add 5 mL of 50% ethanol to dissolve, filter through a 0.22 μm microporous membrane, and inject the filtrate into a preparative high-performance liquid chromatograph, the mobile phase is absolute ethanol-water (40:60), and the flow rate is 10 mL / min, collect the effluent from the chromatographic peak corresponding to Tuimoside C, recover the solvent under reduced pressure, and freeze-dry to obtain 190.4 mg of transparent Tuifumoside A crystal.
[0055]Orbitrap Elite mass spectrometer (Thermo Scientific, Bremen, Germany) was used to detect the molecular weight of the prepared tulimoside A. See Figure 5 . The quasi-molecular ion peak of HR-ESI-MS m / z 1363.6233[M-H] - (calculated value: 1364.6249), molecular formula: C 64 h 100 o 31 .
[0056] An Agilent DD2-600 nuclear magnetic resonance instrument (TMS as internal standard, DMSO-D 6 as a solvent) to detect the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com